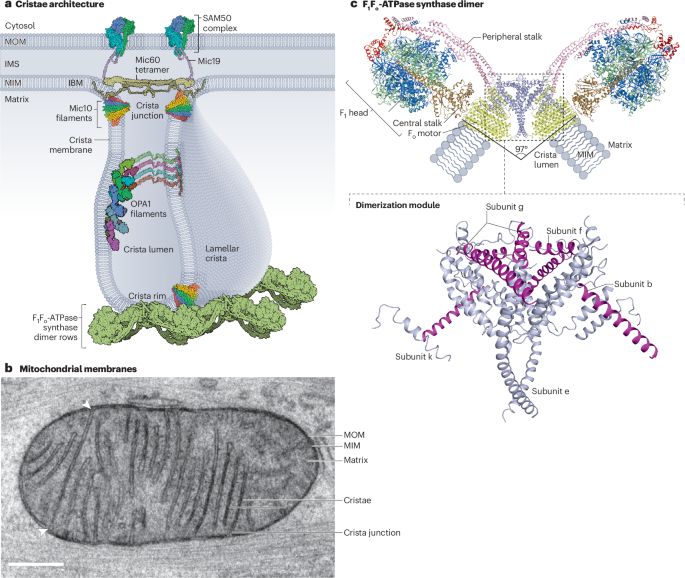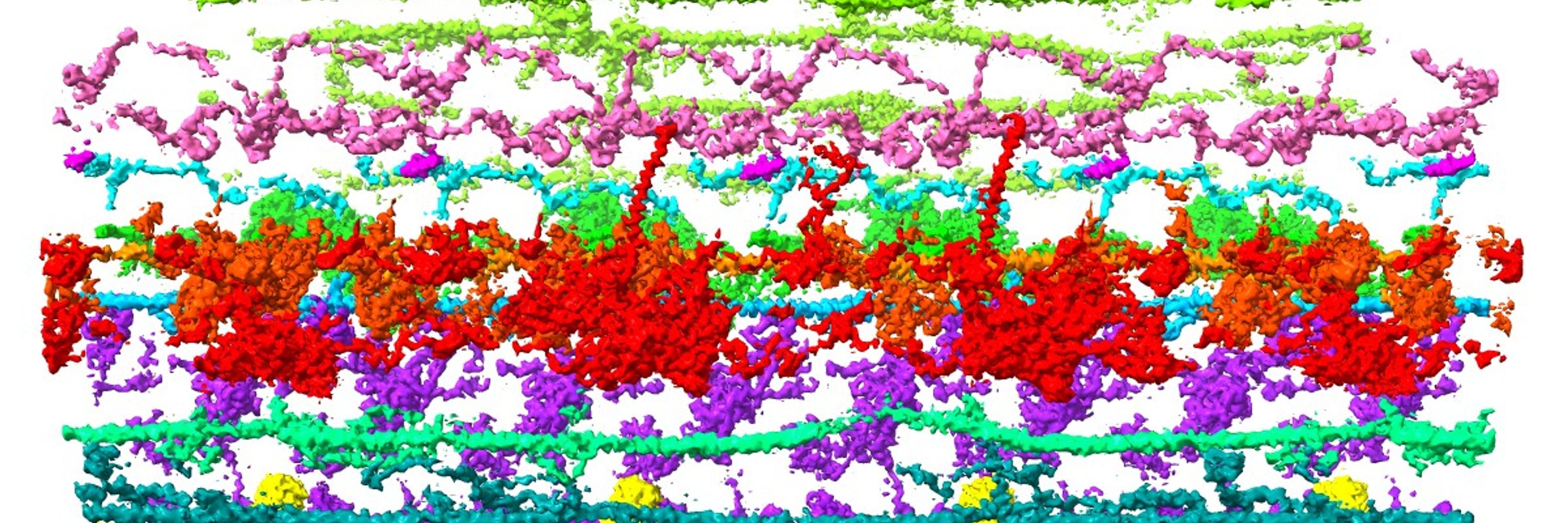
Have been working on motor protein, microtubule, and cilia.
Recently also working with membrane proteins.
Associate Editorial Board member of Cytoskeleton Journal.
Lab website: https://tinyurl.com/22c3eymy
Happy reading!
#cilia #flagella
journals.biologists.com/jcs/issue/13...

Happy reading!
#cilia #flagella
journals.biologists.com/jcs/issue/13...
Hope we didn’t miss anything important - apologies if we did!
#cilia #ciliopathies #proteomics
@theracilproject.bsky.social @for5547.bsky.social
tinyurl.com/yc8ztn5v

Hope we didn’t miss anything important - apologies if we did!
#cilia #ciliopathies #proteomics
@theracilproject.bsky.social @for5547.bsky.social
tinyurl.com/yc8ztn5v

journals.biologists.com/jcs/article/...

journals.biologists.com/jcs/article/...


www.biorxiv.org/content/10.1...

www.biorxiv.org/content/10.1...

www.biorxiv.org/content/10.1...
www.biorxiv.org/content/10.1...
➡️ www.nature.com/articles/s41...
Congrats to all authors from me and Anthony 🎉 @dunnschool.bsky.social Check out this animation made by talented PhD student @matthew-batisio.bsky.social 😆
➡️ www.nature.com/articles/s41...
Congrats to all authors from me and Anthony 🎉 @dunnschool.bsky.social Check out this animation made by talented PhD student @matthew-batisio.bsky.social 😆
www.nature.com/articles/s41...
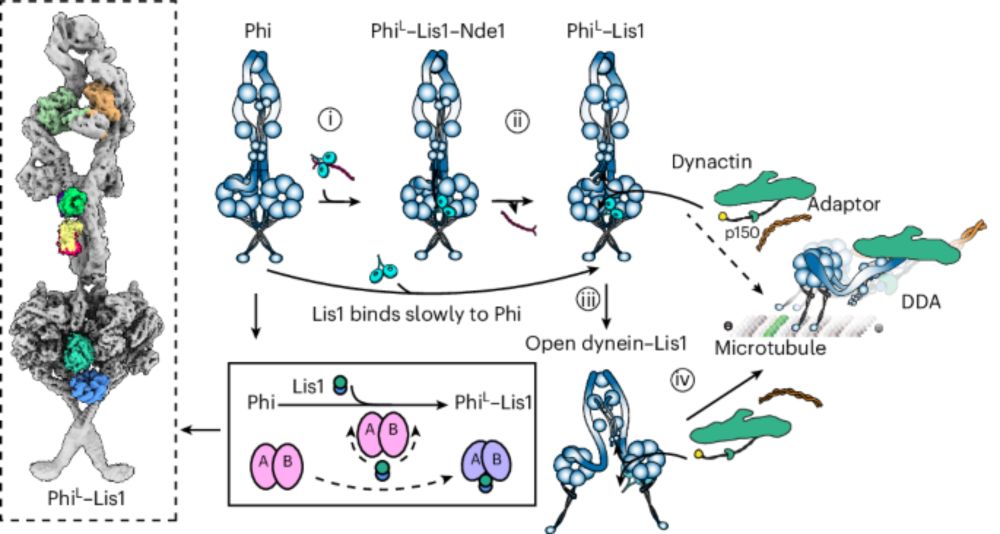
www.nature.com/articles/s41...


www.nature.com/collections/...

www.nature.com/collections/...
We are seeking a new editor with expertise in microscopy, imaging technology, and/or cell biology. The position is available in NYC/Jersey City or Shanghai/Beijing on a hybrid working model.
Apply by August 3!
springernature.wd3.myworkdayjobs.com/SpringerNatu...

We report how human outer kinetochore complexes Ndc80 and Ska form cooperative oligomers, that together stabilise microtubule ends against shortening.
www.biorxiv.org/content/10.1...
Key results below: (1/7)
We report how human outer kinetochore complexes Ndc80 and Ska form cooperative oligomers, that together stabilise microtubule ends against shortening.
www.biorxiv.org/content/10.1...
Key results below: (1/7)
www.nature.com/articles/s41...

www.nature.com/articles/s41...
By combining #cryoEM with #AlphaFold3 modelling, we propose that norovirus NS3 forms a transmembrane RNA translocase.
This could have big implications for our understanding of viral replication & assembly (🧵)
www.biorxiv.org/content/10.1...
By combining #cryoEM with #AlphaFold3 modelling, we propose that norovirus NS3 forms a transmembrane RNA translocase.
This could have big implications for our understanding of viral replication & assembly (🧵)
www.biorxiv.org/content/10.1...
Want to know everything tubulin assembly in trypanosome flagella and other microtubules? Here is the link:
journals.biologists.com/jcs/article/...
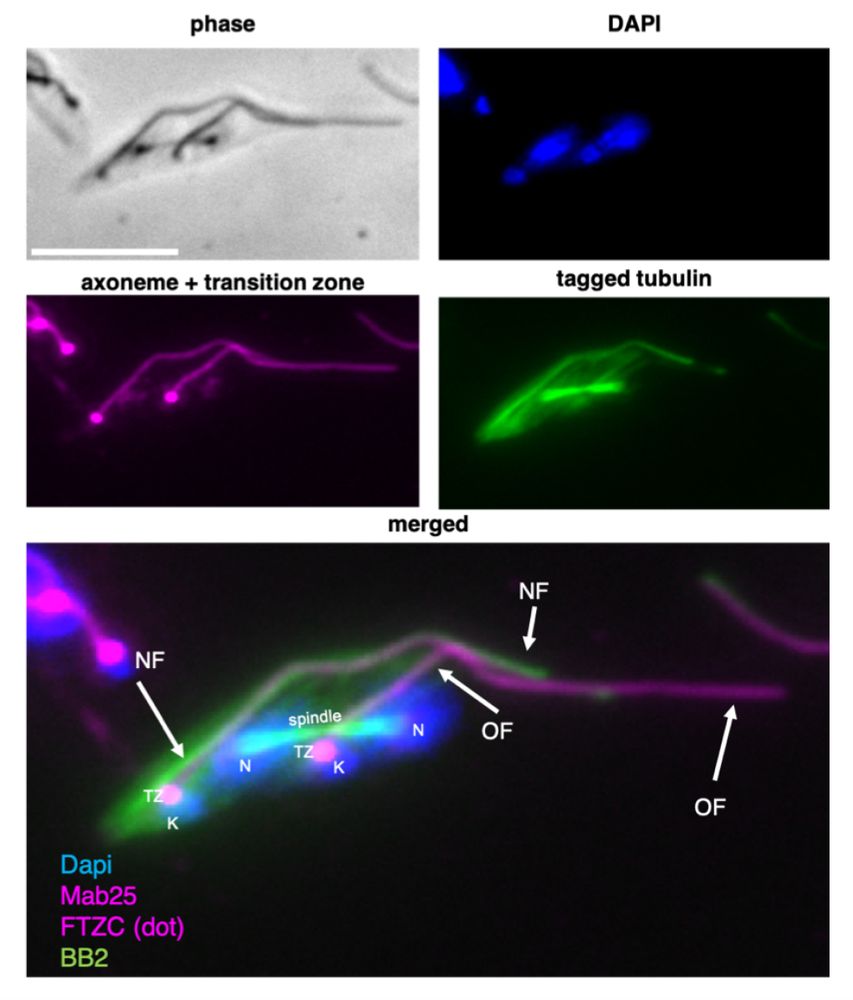
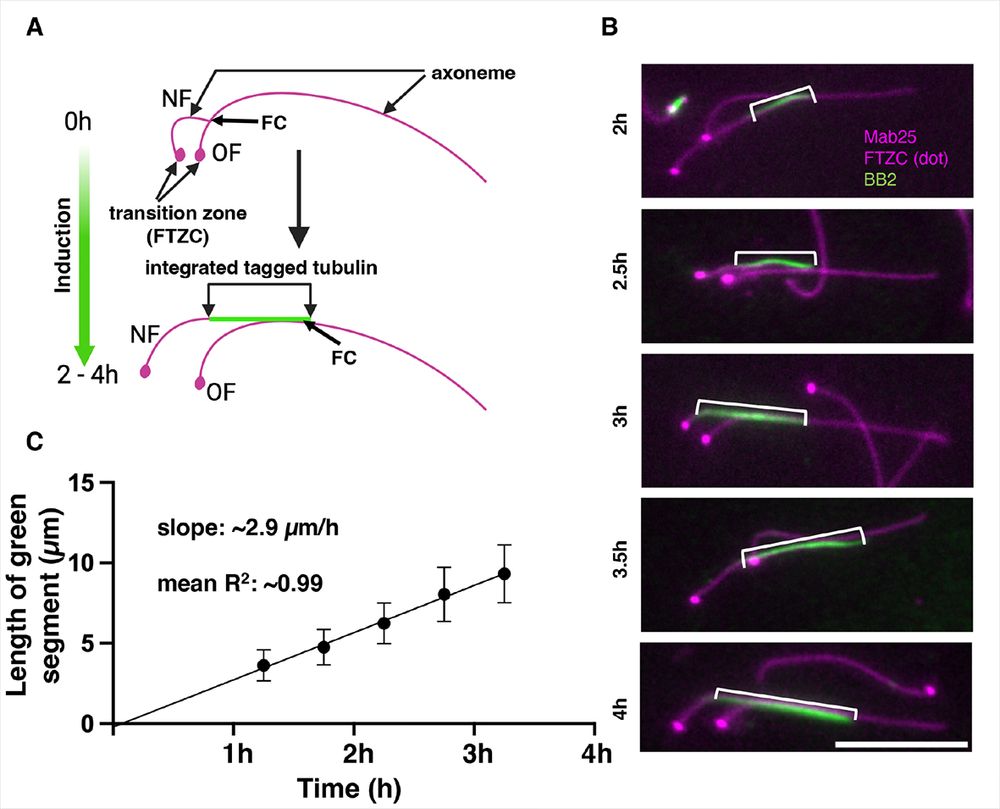
Want to know everything tubulin assembly in trypanosome flagella and other microtubules? Here is the link:
journals.biologists.com/jcs/article/...

Canada Excellence Research Chair (C$5 or C$10M, 8 yr budget)
U Toronto, Dept of Biochem/LabMedPath
*Was just told they're looking for a structural biologist using cryoEM* (related to infectious disease?)
Are you an established PI ready to move to 🇨🇦?
research.utoronto.ca/funding-oppo...
Canada Excellence Research Chair (C$5 or C$10M, 8 yr budget)
U Toronto, Dept of Biochem/LabMedPath
*Was just told they're looking for a structural biologist using cryoEM* (related to infectious disease?)
Are you an established PI ready to move to 🇨🇦?
research.utoronto.ca/funding-oppo...