
Postdoc at @boultonlab.bsky.social, before that Research Fellow at the University of Sussex with keithcaldecott.bsky.social.





www.nature.com/articles/s41...
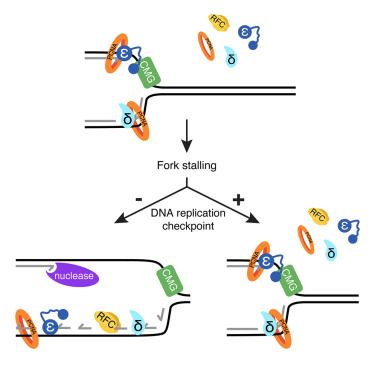
We discovered that PARP inhibitors 💊 trigger histone eviction from the chromatin and this creates a hidden vulnerability in PARPi resistant tumors.
🧵 (1/8)

www.nature.com/articles/s41...
We discovered that PARP inhibitors 💊 trigger histone eviction from the chromatin and this creates a hidden vulnerability in PARPi resistant tumors.
🧵 (1/8)



Congrats Thijn! 🎉 @thijnbrummel.bsky.social
Full article ➡️ bit.ly/40p4KnK

Congrats Thijn! 🎉 @thijnbrummel.bsky.social
Full article ➡️ bit.ly/40p4KnK

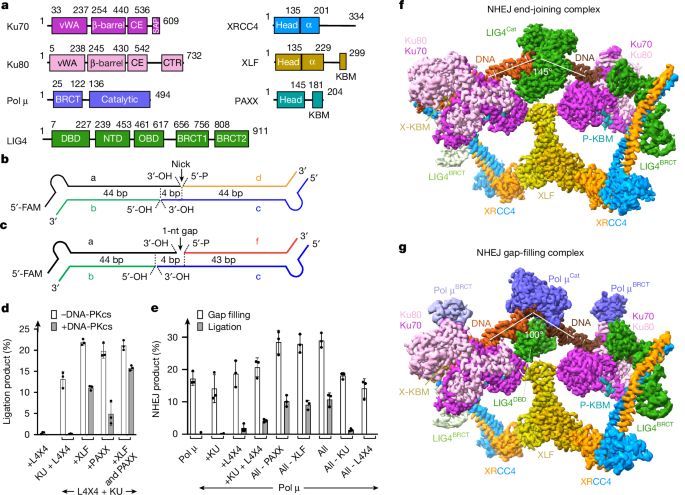

elifesciences.org/reviewed-pre...

elifesciences.org/reviewed-pre...

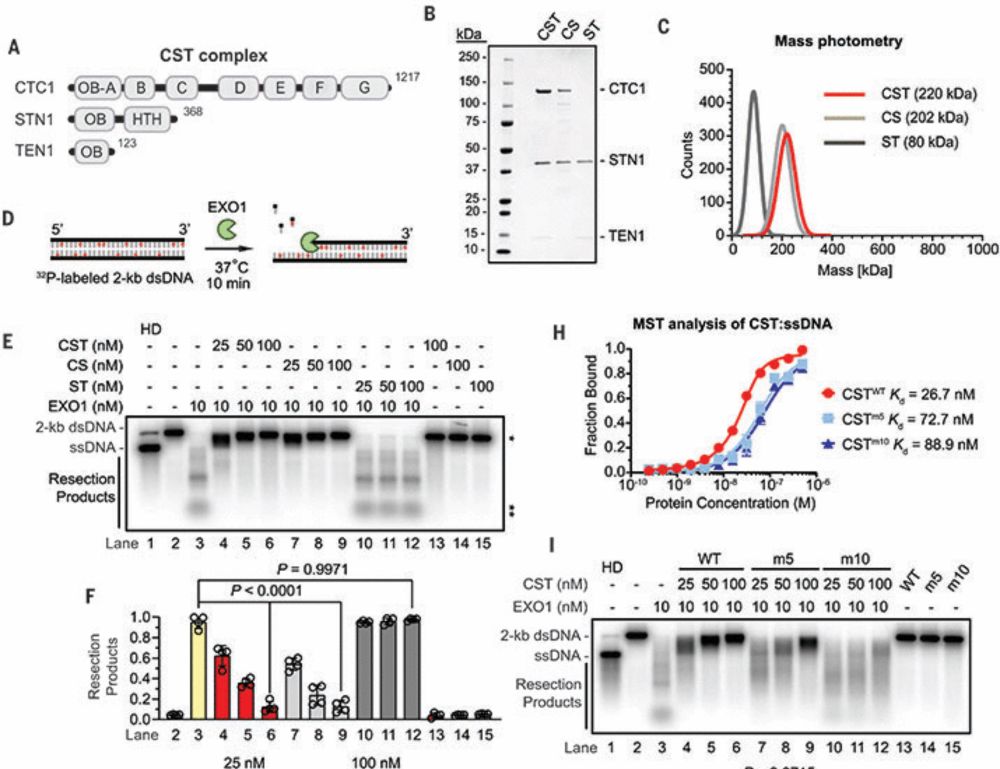
Artios is a biotech company that develops cancer treatments that target DNA damage response pathways.
www.crick.ac.uk/news-and-fea...

Artios is a biotech company that develops cancer treatments that target DNA damage response pathways.
www.crick.ac.uk/news-and-fea...
www.sussex.ac.uk/study/fees-f...
www.sussex.ac.uk/study/fees-f...
my.corehr.com/pls/uoxrecru...
my.corehr.com/pls/uoxrecru...



