#ChemSky 🧪

#ChemSky 🧪
Congratulations! Your PNAS article, "A 5000-fold Increase in the HAT Reactivity of a Nonheme FeIV=O Complex Simply by Replacing Two Pyridines of N4Py Ligand with Pyrazoles", has published and is now available at: www.pnas.org/doi/10.1073/pnas.2414962122
#ChemSky
Congratulations! Your PNAS article, "A 5000-fold Increase in the HAT Reactivity of a Nonheme FeIV=O Complex Simply by Replacing Two Pyridines of N4Py Ligand with Pyrazoles", has published and is now available at: www.pnas.org/doi/10.1073/pnas.2414962122
#ChemSky
An ultrasound-assisted C-H functionalization of ketones using a KI/tert-butyl hydroperoxide catalytic system enables a synthesis of imidazo[1,2-a]pyridines and imidazo[2,1-b]thiazoles in good yields.

An ultrasound-assisted C-H functionalization of ketones using a KI/tert-butyl hydroperoxide catalytic system enables a synthesis of imidazo[1,2-a]pyridines and imidazo[2,1-b]thiazoles in good yields.

ZnMe2 promotes a direct C2- or C4-selective primary and secondary alkylation of a broad range of pyridines and quinolines using 1,1-diborylalkanes as alkylation sources.

ZnMe2 promotes a direct C2- or C4-selective primary and secondary alkylation of a broad range of pyridines and quinolines using 1,1-diborylalkanes as alkylation sources.
👉 thieme-connect.com/products/ejo...

👉 thieme-connect.com/products/ejo...

A photoinduced intermolecular charge transfer between 1,4-dihydropyridines and N-amidopyridinium salts for the facile C4-functionalization of pyridines

A photoinduced intermolecular charge transfer between 1,4-dihydropyridines and N-amidopyridinium salts for the facile C4-functionalization of pyridines
chemsky 🧪
www.science.org/doi/10.1126/...
chemsky 🧪
www.science.org/doi/10.1126/...
4-Alkyl or aryl substituted Hantzsch 1,4-dihydropyridines are aromatized to the corresponding pyridines in high yields

4-Alkyl or aryl substituted Hantzsch 1,4-dihydropyridines are aromatized to the corresponding pyridines in high yields
Article by R Güdük, N Kehl, C Stavagna, MJ. Tilby, O Turner, A Ruffoni, HP. Caldora & D Leonori
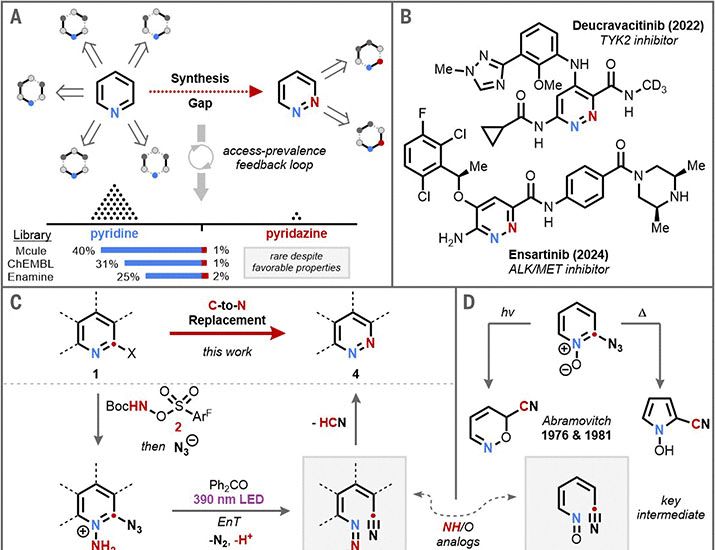
A three-step strategy for the conversion of pyridines into benzonitriles
www.nature.com/articles/s44...
#ChemSky

Article by R Güdük, N Kehl, C Stavagna, MJ. Tilby, O Turner, A Ruffoni, HP. Caldora & D Leonori
A three-step strategy for the conversion of pyridines into benzonitriles
www.nature.com/articles/s44...
#ChemSky
Article by Ning Jiao, Song Song & co-workers
Direct regioselective C-3 halogenation of pyridines
www.nature.com/articles/s44... ($)
#Chemsky

Article by Ning Jiao, Song Song & co-workers
Direct regioselective C-3 halogenation of pyridines
www.nature.com/articles/s44... ($)
#Chemsky
Contrairement à ce que disent certains textes (UpTodate) les dihydro pyridines peuvent ou pas ↘️ l'aldo et le %A/R mais chez elle l'aldo n'est pas basse.
Je n'ai pas encore les urines et Na de 24h ...
1/2
Contrairement à ce que disent certains textes (UpTodate) les dihydro pyridines peuvent ou pas ↘️ l'aldo et le %A/R mais chez elle l'aldo n'est pas basse.
Je n'ai pas encore les urines et Na de 24h ...
1/2



->Nature | More from Lil Dr Glen EcoChat at BigEarthData.ai

#chemistry #openaccess #science

#chemistry #openaccess #science

🔓Check out the #OpenAccess paper behind the cover by Vandana Srivastava, Sundaram Singh & co. at IIT Varanasi reporting the photocatalytic synthesis of imidazo[1,2-a]pyridines via C(sp)3–H functionalization using ethylarene #orgchem
pubs.rsc.org/en/content/a...

🔓Check out the #OpenAccess paper behind the cover by Vandana Srivastava, Sundaram Singh & co. at IIT Varanasi reporting the photocatalytic synthesis of imidazo[1,2-a]pyridines via C(sp)3–H functionalization using ethylarene #orgchem
pubs.rsc.org/en/content/a...
www.mdpi.com/2673-4583/18...
By David Calderón-Rangel et al.
From the 29th International Electronic Conference on Synthetic Organic Chemistry
#Imidazopyridine #UltrasoundSynthesis
www.mdpi.com/2673-4583/18...
By David Calderón-Rangel et al.
From the 29th International Electronic Conference on Synthetic Organic Chemistry
#Imidazopyridine #UltrasoundSynthesis

