prev. PhD with Prof. Edward Lemke @EMBL & @uni_mainz
creating synthetic organelles #condensates #synbio
He joins EMBL from @mpi-cbg.de in Dresden. He is also Professor of Molecular Biology @tudresden.bsky.social, and was a group leader at EMBL Heidelberg from 1993 to 1999.
www.embl.org/news/people-...

He joins EMBL from @mpi-cbg.de in Dresden. He is also Professor of Molecular Biology @tudresden.bsky.social, and was a group leader at EMBL Heidelberg from 1993 to 1999.
www.embl.org/news/people-...


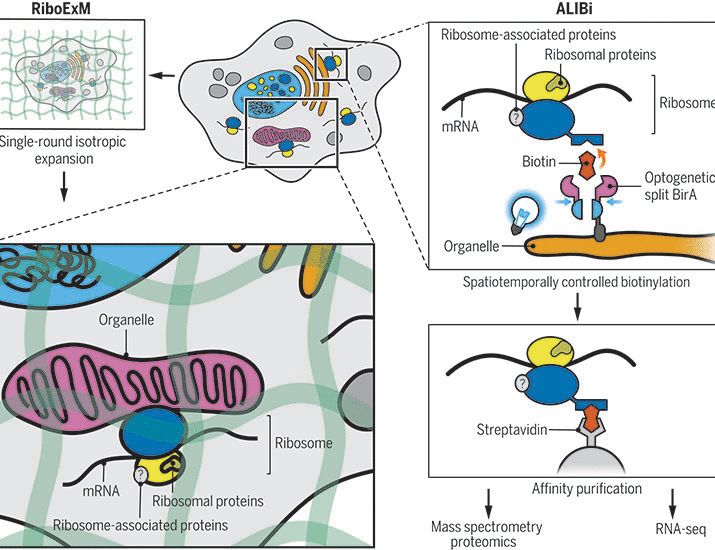
rdcu.be/eq975
rdcu.be/eq975
A huge thank you to our incredible team—this would not have been possible without your dedication and talent. 🙌 @dfg.de
#ClustersOfExcellence #SCALEcluster 🧵

Passionate about enzyme engineering or genetic code expansion? 🧬 Our lab is hiring a PhD student!
🔬 Exciting interdisciplinary research
🤝 Engaging environment 🌱
📄 Does this sound like you, apply now! ⏳🔗
📢 Know someone? Share this! 🔄
jobs.uzh.ch/job-vacancie...
Passionate about enzyme engineering or genetic code expansion? 🧬 Our lab is hiring a PhD student!
🔬 Exciting interdisciplinary research
🤝 Engaging environment 🌱
📄 Does this sound like you, apply now! ⏳🔗
📢 Know someone? Share this! 🔄
jobs.uzh.ch/job-vacancie...
Yesterday, we participated in the #Mainz Carnival alongside the @sfb1551.bsky.social! This was an amazing experience, and with over 600,000 people watching, it was a fantastic way to show how fun science can be!
You can find the recording here, starting at 45:50 👉 shorturl.at/41alV


Yesterday, we participated in the #Mainz Carnival alongside the @sfb1551.bsky.social! This was an amazing experience, and with over 600,000 people watching, it was a fantastic way to show how fun science can be!
You can find the recording here, starting at 45:50 👉 shorturl.at/41alV


Yesterday, we participated in the #Mainz Carnival alongside the @sfb1551.bsky.social! This was an amazing experience, and with over 600,000 people watching, it was a fantastic way to show how fun science can be!
You can find the recording here, starting at 45:50 👉 shorturl.at/41alV
We decided to call them TIGR-Tas systems 🐯
We decided to call them TIGR-Tas systems 🐯

pubs.acs.org/doi/full/10....

pubs.acs.org/doi/full/10....
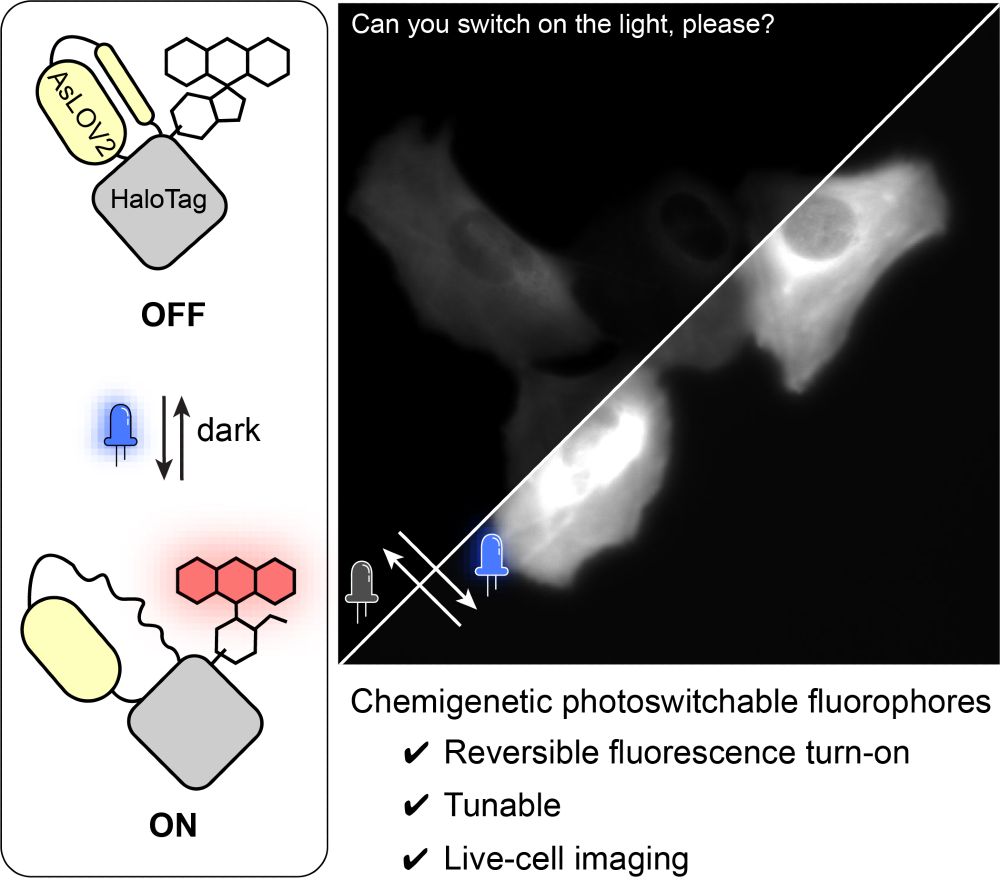
www.biorxiv.org/content/10.1...
#Chembio #FluorescenceFriday

www.biorxiv.org/content/10.1...
#Chembio #FluorescenceFriday
Confinement and catalysis within de novo designed peptide barrels
doi.org/10.1021/jacs...
(Journal of the American Chemical Society)

Confinement and catalysis within de novo designed peptide barrels
doi.org/10.1021/jacs...
(Journal of the American Chemical Society)

If you know someone who might be interested, please share this post with them! 🎓 Thank you in advance!
Our partner institutions are @Goethe University Frankfurt, @unimainz.bsky.social, @Frankfurt Institute of Advanced Studies and @mpibp.bsky.social
If you know someone who might be interested, please share this post with them! 🎓 Thank you in advance!
Our partner institutions are @Goethe University Frankfurt, @unimainz.bsky.social, @Frankfurt Institute of Advanced Studies and @mpibp.bsky.social
#Ub #PQC #condensates

#Ub #PQC #condensates
and the team, and many thanks to the lab of Jan-Michael Peters for support!
We found that the interphase and mitotic chromatin loop organization have more in common than previously thought:
In both stages big loops are built first, small ones second
@wanluzhang.bsky.social

and the team, and many thanks to the lab of Jan-Michael Peters for support!
We found that the interphase and mitotic chromatin loop organization have more in common than previously thought:
In both stages big loops are built first, small ones second
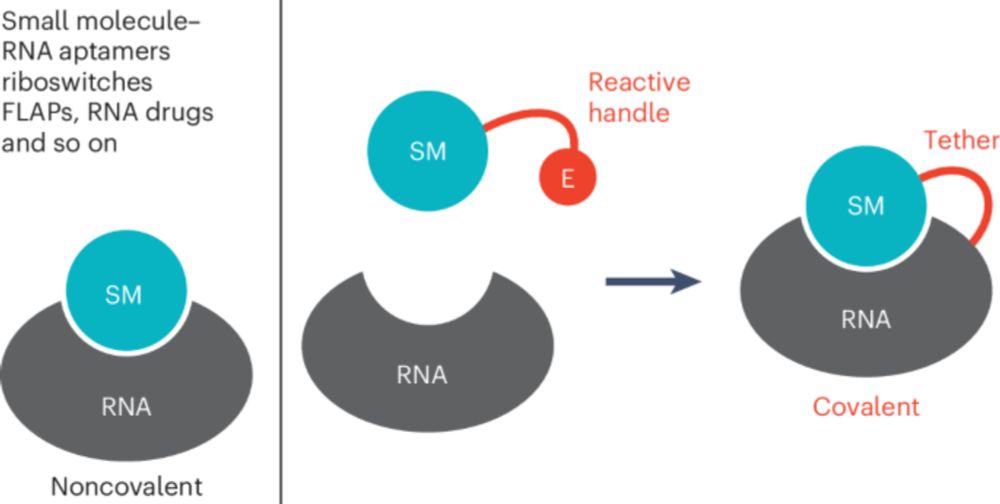
www.biorxiv.org/content/10.1...
www.biorxiv.org/content/10.1...