aacrjournals.org/cancerres/ar...
aacrjournals.org/cancerres/ar...
Be sure to also check out her recent paper in Cancer Research elucidating genetic mechanisms of immune suppression and immunotherapy resistance in prostate cancer.
aacrjournals.org/cancerres/ar...
Be sure to also check out her recent paper in Cancer Research elucidating genetic mechanisms of immune suppression and immunotherapy resistance in prostate cancer.
aacrjournals.org/cancerres/ar...
aacrjournals.org/cancerdiscov...
aacrjournals.org/cancerdiscov...

Be sure to check out her poster # B049 today from 6-9pm!

Be sure to check out her poster # B049 today from 6-9pm!
www.pnas.org/doi/10.1073/...
www.pnas.org/doi/10.1073/...
rdcu.be/eGUtj

rdcu.be/eGUtj

If you see her at the conference, be sure to ask her about her work on PDAC cachexia, bioRxiv link below!
biorxiv.org/content/10.1...






www.cell.com/developmenta...
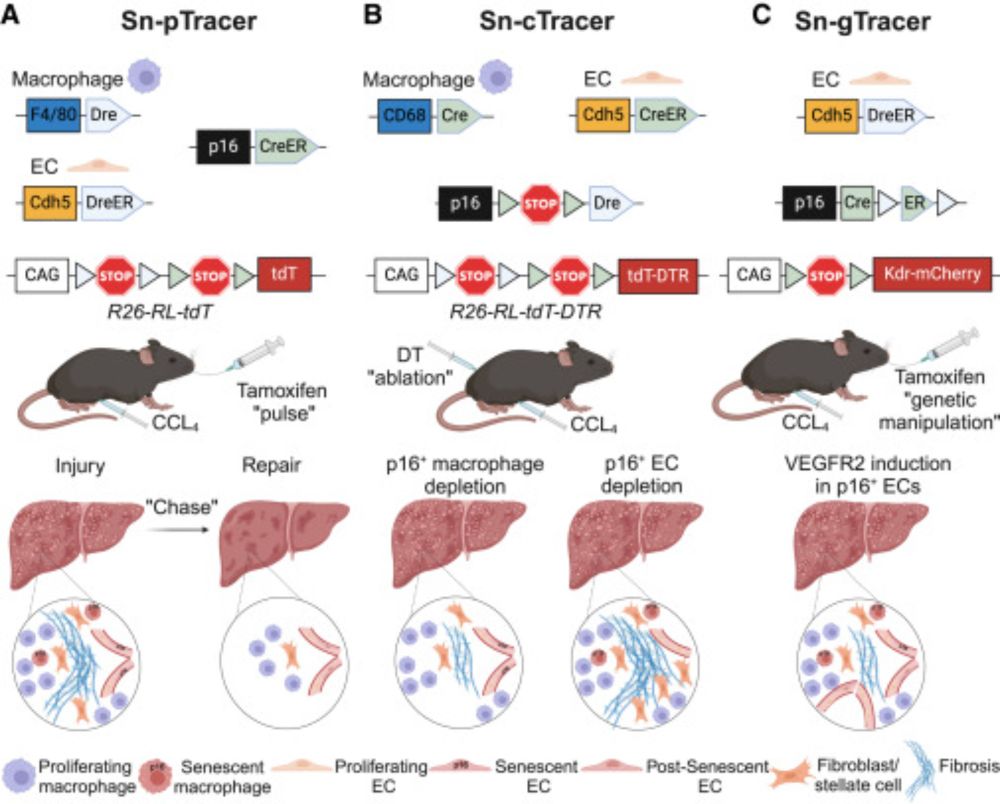
www.cell.com/developmenta...
www.cell.com/trends/cance...
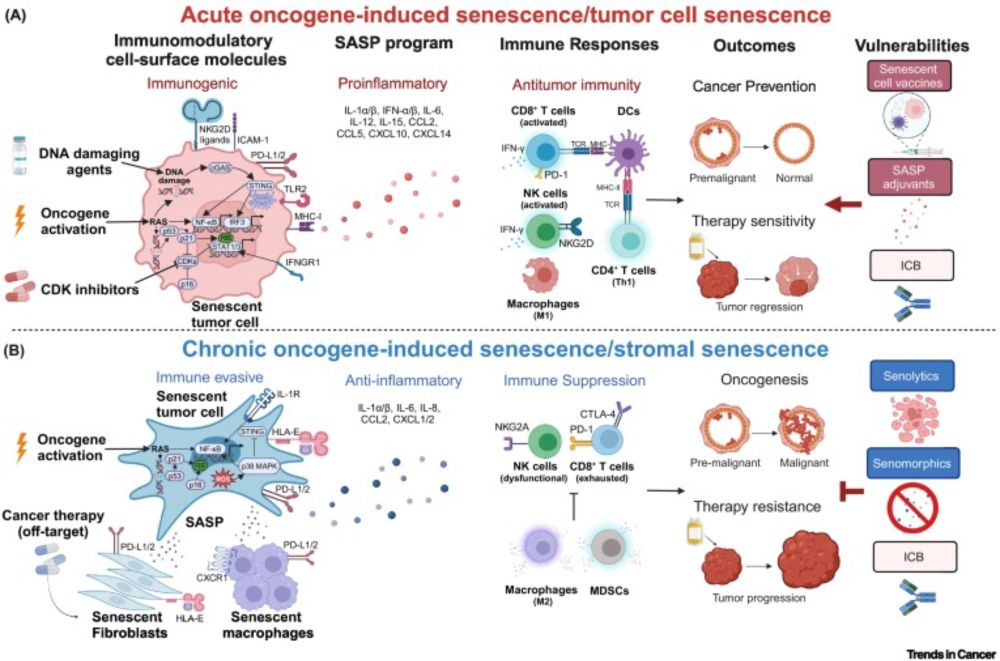
www.cell.com/trends/cance...

We reveal a strategy to engineer extrachromosomal DNA (ecDNA) amplifications in cells & mice. Let's dive in! Let’s dive in! 🧵👇
www.nature.com/articles/s41...
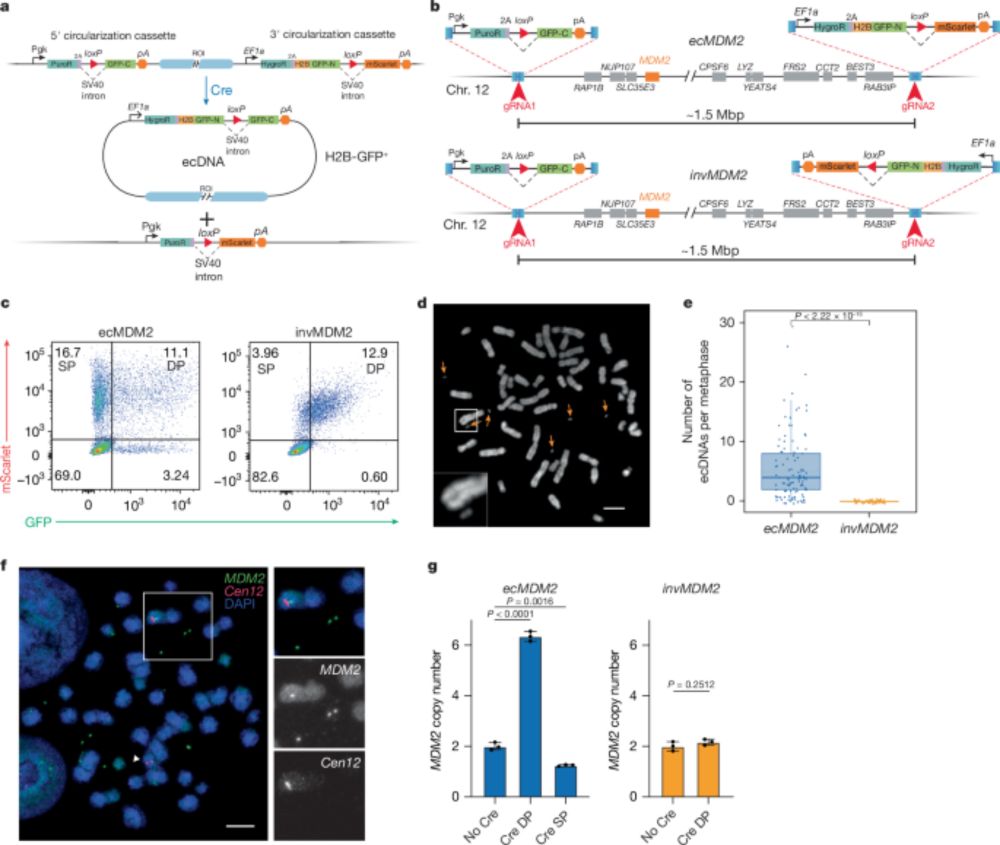
We reveal a strategy to engineer extrachromosomal DNA (ecDNA) amplifications in cells & mice. Let's dive in! Let’s dive in! 🧵👇
www.nature.com/articles/s41...

www.nature.com/articles/s41...
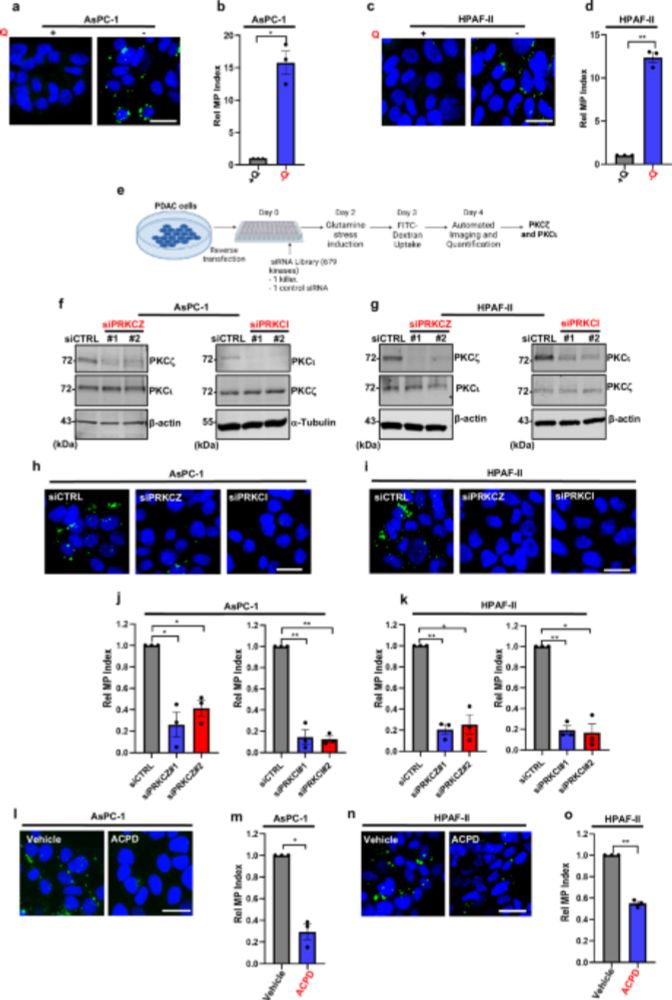
www.nature.com/articles/s41...