🇸🇬🇨🇦🇦🇺
This time looking at the GAMECHANGER trial:
clarityinitiative.org/commentaries...
@erinmccreary.bsky.social with Ahmad Mourad
@gurujosh.bsky.social
#IDSky

This time looking at the GAMECHANGER trial:
clarityinitiative.org/commentaries...
@erinmccreary.bsky.social with Ahmad Mourad
@gurujosh.bsky.social
#IDSky
#IDSky #SNAP_trial
www.sciencedirect.com/science/arti...

#IDSky #SNAP_trial
www.sciencedirect.com/science/arti...

www.clinicalmicrobiologyandinfection.org/article/S119...
We use the BALANCE and CAMERA2 trials as case studies to illustrate the use of the different methods available for analysis of hierarchical composite endpoints.
#IDSky @steventong.bsky.social

www.clinicalmicrobiologyandinfection.org/article/S119...
We use the BALANCE and CAMERA2 trials as case studies to illustrate the use of the different methods available for analysis of hierarchical composite endpoints.
#IDSky @steventong.bsky.social
https://ow.ly/HZao50XwVYg
#IDSky #clinmicro

https://ow.ly/HZao50XwVYg
#IDSky #clinmicro
unimelb.zoom.us/j/8944229688...
@thedohertyinst.bsky.social

unimelb.zoom.us/j/8944229688...
@thedohertyinst.bsky.social
Accounting for non-adherence to assigned antibiotic treatment duration for bloodstream infection (BALANCE): a post-hoc analysis of a randomised clinical trial
www.thelancet.com/journals/lan...

Accounting for non-adherence to assigned antibiotic treatment duration for bloodstream infection (BALANCE): a post-hoc analysis of a randomised clinical trial
www.thelancet.com/journals/lan...
authors.elsevier.com/a/1m5He5E-Uo...
@steventong.bsky.social #IDSky
authors.elsevier.com/a/1m5He5E-Uo...
@steventong.bsky.social #IDSky
More info here: forms.gle/H9TXEMkwM1cb...
Please spread far and wide!
@steventong.bsky.social #IDSky
More info here: forms.gle/H9TXEMkwM1cb...
Please spread far and wide!
@steventong.bsky.social #IDSky
Joining the X-odus: Contrasting perspectives on whether infection specialists should leave X (formerly Twitter)
That I'm sharing it here belies where I stand on the issue
www.cmi-comms.org/article/S295... #IDSky
#IDSky #Clinmicro

#IDSky #Clinmicro
doi.org/10.1016/j.cm...
@steventong.bsky.social #IDSky

doi.org/10.1016/j.cm...
@steventong.bsky.social #IDSky
Compared to similar non-trial population w/ MRSA bacteremia:
-No difference in 🪦, BUT
-non-trial had less following of quality metrics (see ⬇️)
#IDSky
jamanetwork.com/journals/jam...
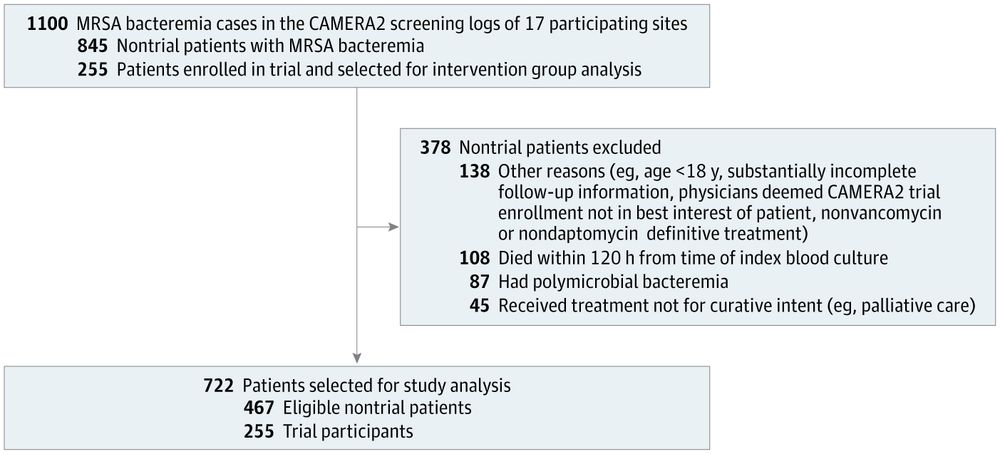
Compared to similar non-trial population w/ MRSA bacteremia:
-No difference in 🪦, BUT
-non-trial had less following of quality metrics (see ⬇️)
#IDSky
jamanetwork.com/journals/jam...
✅ Just Accepted
#IDSky

We discuss this trial, which evaluated the impact of different recruitment letter formats on RCT enrolment: jamanetwork.com/journals/jam...

We discuss this trial, which evaluated the impact of different recruitment letter formats on RCT enrolment: jamanetwork.com/journals/jam...
doi.org/10.1093/cid/...
We provide a conceptual overview of HCEs, explain the different associated target parameters and analytic methods.
@steventong.bsky.social @gurujosh.bsky.social

doi.org/10.1093/cid/...
We provide a conceptual overview of HCEs, explain the different associated target parameters and analytic methods.
@steventong.bsky.social @gurujosh.bsky.social
@seanong.bsky.social @gurujosh.bsky.social @steventong.bsky.social and colleagues
Open Access #IDSky #MedSky
academic.oup.com/cid/advance-...
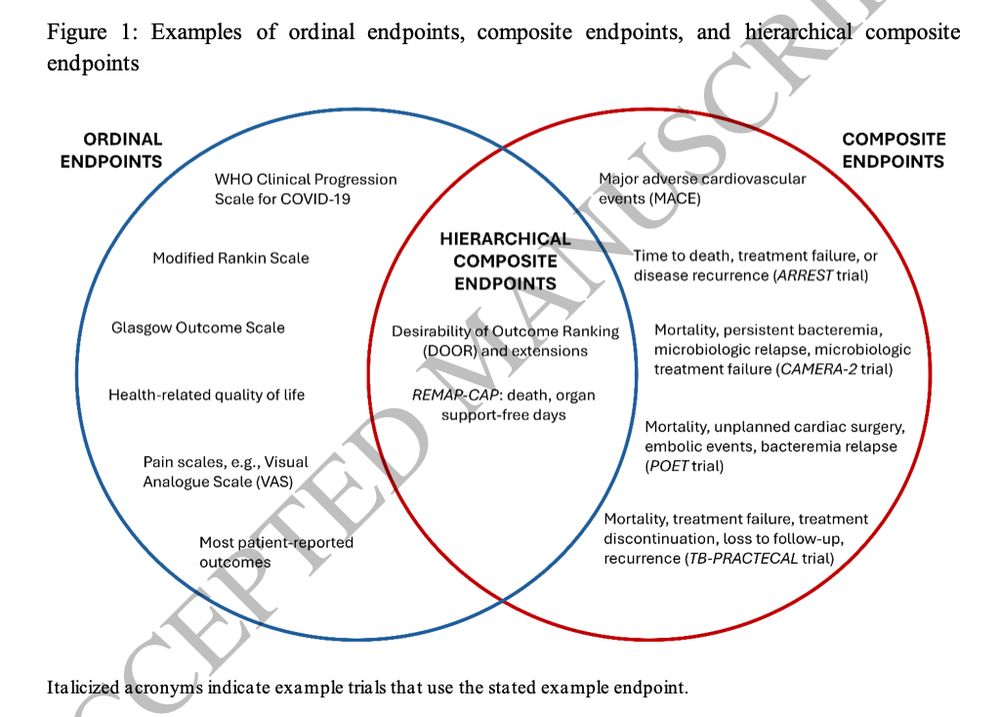
@seanong.bsky.social @gurujosh.bsky.social @steventong.bsky.social and colleagues
Open Access #IDSky #MedSky
academic.oup.com/cid/advance-...
jamanetwork.com/journals/jam...
#IDSky

jamanetwork.com/journals/jam...
#IDSky
These estimates are lower than those in previous literature, which may be due to robust adjustment for confounding
doi.org/10.1093/cid/...
@cidjournal.bsky.social
These estimates are lower than those in previous literature, which may be due to robust adjustment for confounding
doi.org/10.1093/cid/...
@cidjournal.bsky.social
#IDSky
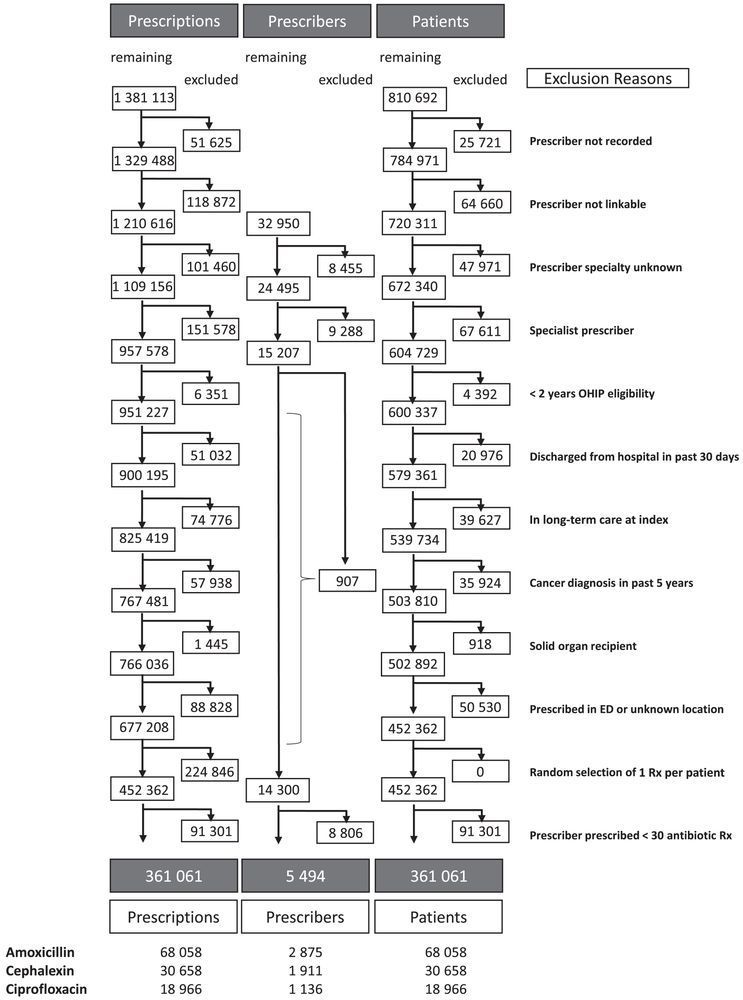
#IDSky
@connorprosty.bsky.social @seanong.bsky.social &c
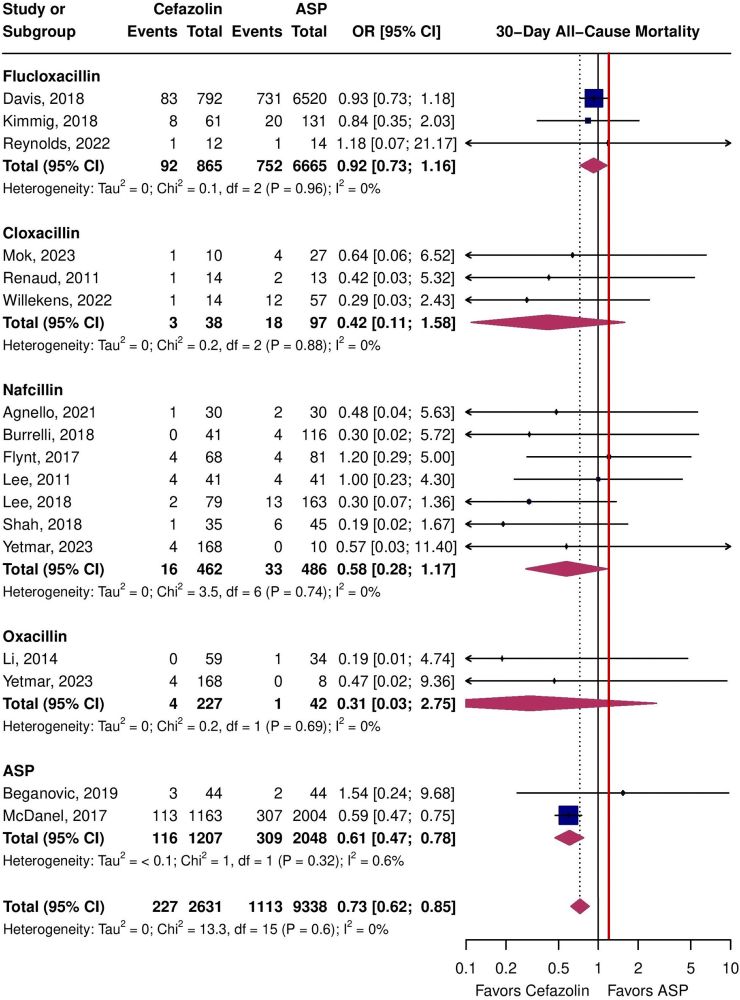
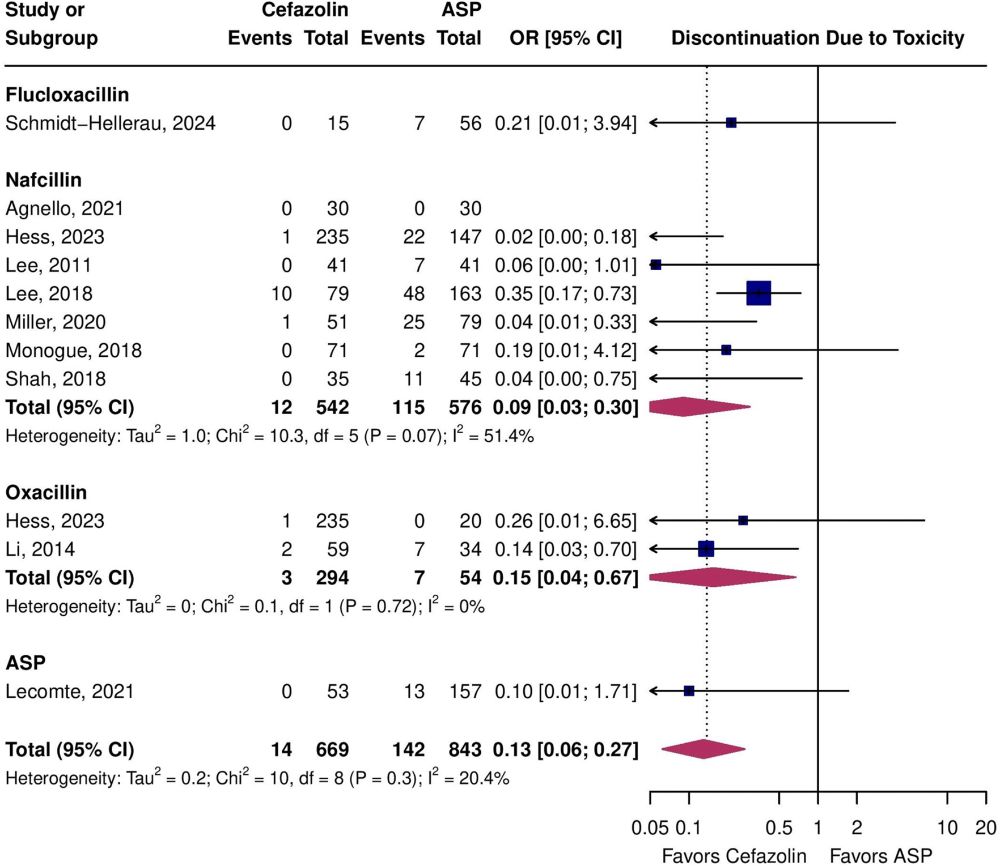
Cefaz more effective, safer #idsky
www.clinicalmicrobiologyandinfection.com/article/S119... 🔓
@connorprosty.bsky.social @seanong.bsky.social &c
Cefaz more effective, safer #idsky
www.clinicalmicrobiologyandinfection.com/article/S119... 🔓
@steventong.bsky.social #IDSky
doi.org/10.1016/j.cm...
@steventong.bsky.social #IDSky
doi.org/10.1016/j.cm...
In first collaboration with #Breakpoints, we unpack new results from the S. aureus SNAP trial platform with @gurujosh.bsky.social & @steventong.bsky.social. Listen here:
#IDSky #Clinmicro #MedSky #PharmSky

In first collaboration with #Breakpoints, we unpack new results from the S. aureus SNAP trial platform with @gurujosh.bsky.social & @steventong.bsky.social. Listen here:
#IDSky #Clinmicro #MedSky #PharmSky
Just 1/4 blood cultures positive (≥2 sets drawn) or early clearance → low IE probability. Persistent bacteremia or 4/4 bottles ↑ pretest probability
Simple bedside clues to sharpen your endocarditis game. #IDSky #EMIMCC
jamanetwork.com/journals/jam...
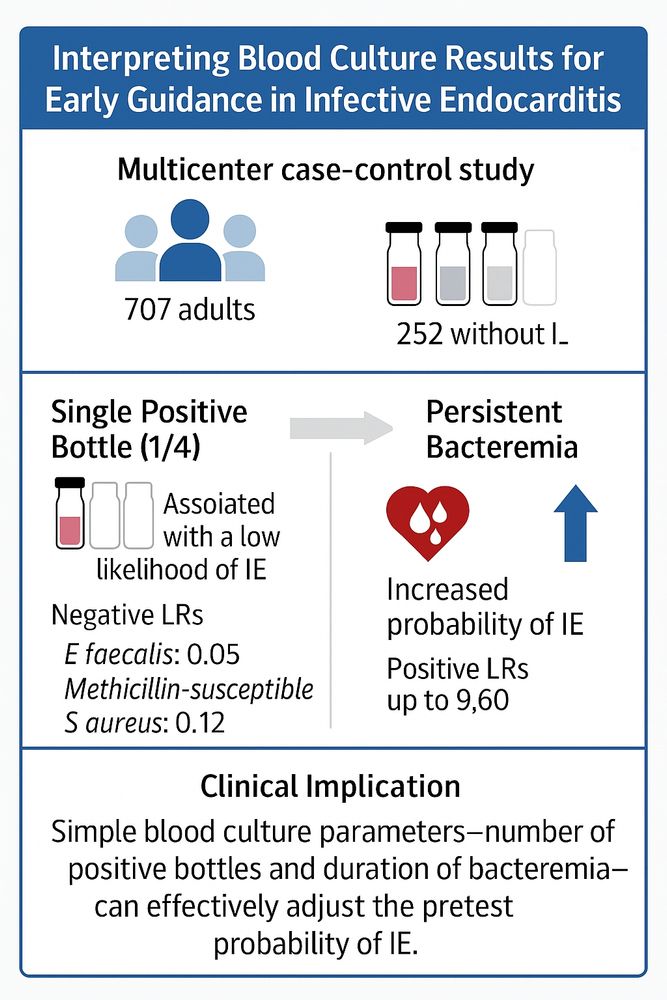
Just 1/4 blood cultures positive (≥2 sets drawn) or early clearance → low IE probability. Persistent bacteremia or 4/4 bottles ↑ pretest probability
Simple bedside clues to sharpen your endocarditis game. #IDSky #EMIMCC
jamanetwork.com/journals/jam...


