
#IDSky #SNAPtrial
Also see/hear our Communicable podcast episode on this at communicable.transistor.fm/episodes/com...
What were your top 10? Any thoughts about what I missed welcome!
#IDSky #Top10papers

Also see/hear our Communicable podcast episode on this at communicable.transistor.fm/episodes/com...
What were your top 10? Any thoughts about what I missed welcome!
#IDSky #Top10papers
#IDSky

#IDSky
This time looking at the GAMECHANGER trial:
clarityinitiative.org/commentaries...
@erinmccreary.bsky.social with Ahmad Mourad
@gurujosh.bsky.social
#IDSky

This time looking at the GAMECHANGER trial:
clarityinitiative.org/commentaries...
@erinmccreary.bsky.social with Ahmad Mourad
@gurujosh.bsky.social
#IDSky
#flutracking #COVID #rhinovirus #IDSky
academic.oup.com/jid/advance-...

#flutracking #COVID #rhinovirus #IDSky
academic.oup.com/jid/advance-...
@criticalcarereviews.com

Join us! 🌱
#IDSky #MedSky #MedEd
www.cmi-comms.org/article/S295...
Join us! 🌱
#IDSky #MedSky #MedEd
www.cmi-comms.org/article/S295...
The interim CDC will now move from under the Department of Health, Ageing, and Disability to an independent entity as of 1 Jan 2026!
#auspol #idepi #episky #medsky #idsky
Reframes #academia as an infection for which the causative organism may be less easy to define, but that can be just as insidious on affected individuals (clinical academics)!
#AcademicSky #MedSky 🧪 @cidjournal.bsky.social

Buzzsprout - www.buzzsprout.com/1740310/epis...
Apple - podcasts.apple.com/gb/podcast/i...
Spotify - open.spotify.com/episode/588l...

@#IDSky
@#IDSky
doi.org/10.1186/s128...
#idsky #MedSky #HepSky #LiverSky
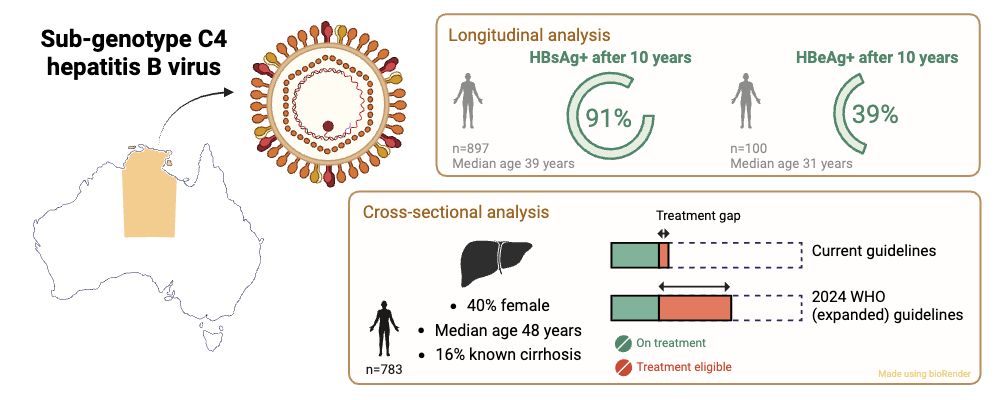
doi.org/10.1186/s128...
#idsky #MedSky #HepSky #LiverSky
doi.org/10.1093/cid/...
We provide a conceptual overview of HCEs, explain the different associated target parameters and analytic methods.
@steventong.bsky.social @gurujosh.bsky.social

doi.org/10.1093/cid/...
We provide a conceptual overview of HCEs, explain the different associated target parameters and analytic methods.
@steventong.bsky.social @gurujosh.bsky.social
Hosted by Josh Davis @gurujosh.bsky.social
Erin McCreary @erinmccreary.bsky.social
Emily McDonald @dremilymcd.bsky.social
Listen here:
#IDSky #Clinmicro #MedSky

Hosted by Josh Davis @gurujosh.bsky.social
Erin McCreary @erinmccreary.bsky.social
Emily McDonald @dremilymcd.bsky.social
Listen here:
#IDSky #Clinmicro #MedSky
Hosted by Marc Bonten @marcbonten.bsky.social
Josh Davis @gurujosh.bsky.social
Erin McCreary @erinmccreary.bsky.social
Emily McDonald @dremilymcd.bsky.social
Listen here:
#IDSky #Clinmicro #MedSky

Hosted by Marc Bonten @marcbonten.bsky.social
Josh Davis @gurujosh.bsky.social
Erin McCreary @erinmccreary.bsky.social
Emily McDonald @dremilymcd.bsky.social
Listen here:
#IDSky #Clinmicro #MedSky
@gurujosh.bsky.social @dremilymcd.bsky.social @erinmccreary.bsky.social @marcbonten.bsky.social @escmid.bsky.social
#IDSky #clinmicro
@gurujosh.bsky.social @dremilymcd.bsky.social @erinmccreary.bsky.social @marcbonten.bsky.social @escmid.bsky.social
#IDSky #clinmicro

joint effort @escmid.bsky.social Communicable & @sidpharm.bsky.social Breakpoints podcast
podcasts.apple.com/us/podcast/s...

joint effort @escmid.bsky.social Communicable & @sidpharm.bsky.social Breakpoints podcast
podcasts.apple.com/us/podcast/s...
www.cmi-comms.com/article/S295...
www.cmi-comms.com/article/S295...
Whose side do you take?
#IDSky #Clinmicro #MedSky #PharmSky #AMR #ICUSky #Criticalcaresky @marcbonten.bsky.social
www.cmi-comms.com/article/S295...
Whose side do you take?
#IDSky #Clinmicro #MedSky #PharmSky #AMR #ICUSky #Criticalcaresky @marcbonten.bsky.social
www.cmi-comms.com/article/S295...
Huge congrats to Drs. Joshua Davis, Steven Tong, & the global team who made this ambitious trial happen. #IDSky blogs.jwatch.org/hiv-id-obser...

Huge congrats to Drs. Joshua Davis, Steven Tong, & the global team who made this ambitious trial happen. #IDSky blogs.jwatch.org/hiv-id-obser...
A new platform trial for iGAS and iSDSE
@gurujosh.bsky.social @joshosowicki.bsky.social @drmichaelmarks.bsky.social
www.doherty.edu.au/news-events/...
A new platform trial for iGAS and iSDSE
@gurujosh.bsky.social @joshosowicki.bsky.social @drmichaelmarks.bsky.social
www.doherty.edu.au/news-events/...
#IDSky #ESCMIDglobal
@steventong.bsky.social

#IDSky #ESCMIDglobal
@steventong.bsky.social
#IDSky
#IDSky
Cefazolin manufacturers are gonna have to really step up after this (still on shortage restrictions in 🇬🇧 😢)
RIP fluclox 🪦
@gurujosh.bsky.social gave an excellent talk 🥁
#ESCMIDGlobal
Cefazolin manufacturers are gonna have to really step up after this (still on shortage restrictions in 🇬🇧 😢)
RIP fluclox 🪦
@gurujosh.bsky.social gave an excellent talk 🥁
#ESCMIDGlobal

