
Kaessmann Lab
@kaessmannlab.bsky.social
Our research group is interested in the molecular and cellular origins and evolution of vertebrate organs. Tweets by Henrik, usually in the name of our group.
home.kaessmannlab.org
home.kaessmannlab.org
Pinned
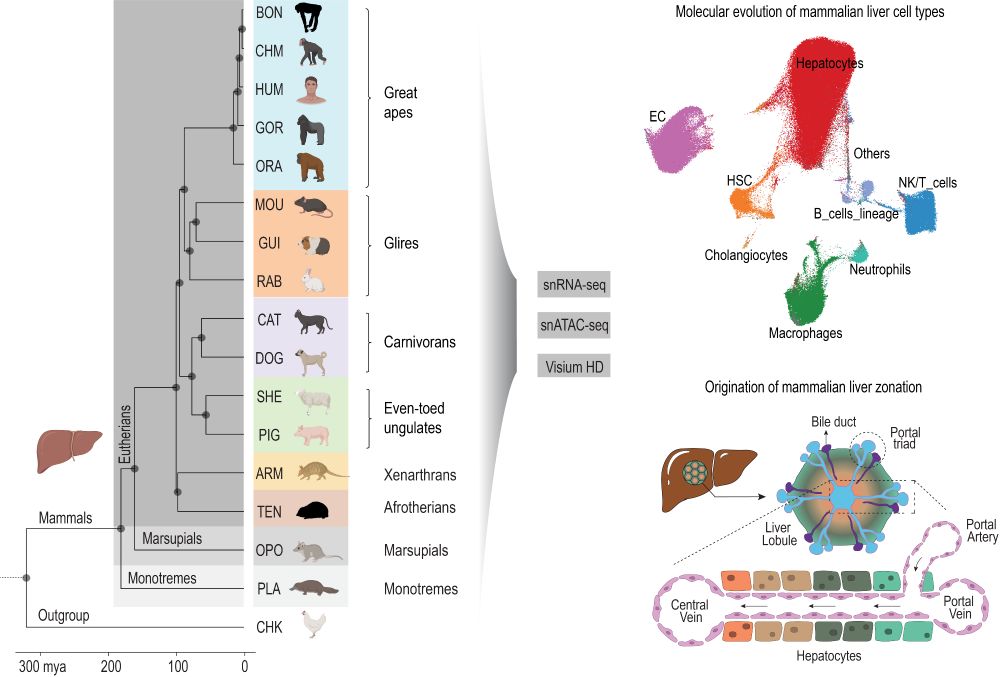
We are thrilled to share our new preprint entitled “The origin and molecular evolution of the mammalian liver cell architecture” www.biorxiv.org/content/10.1...
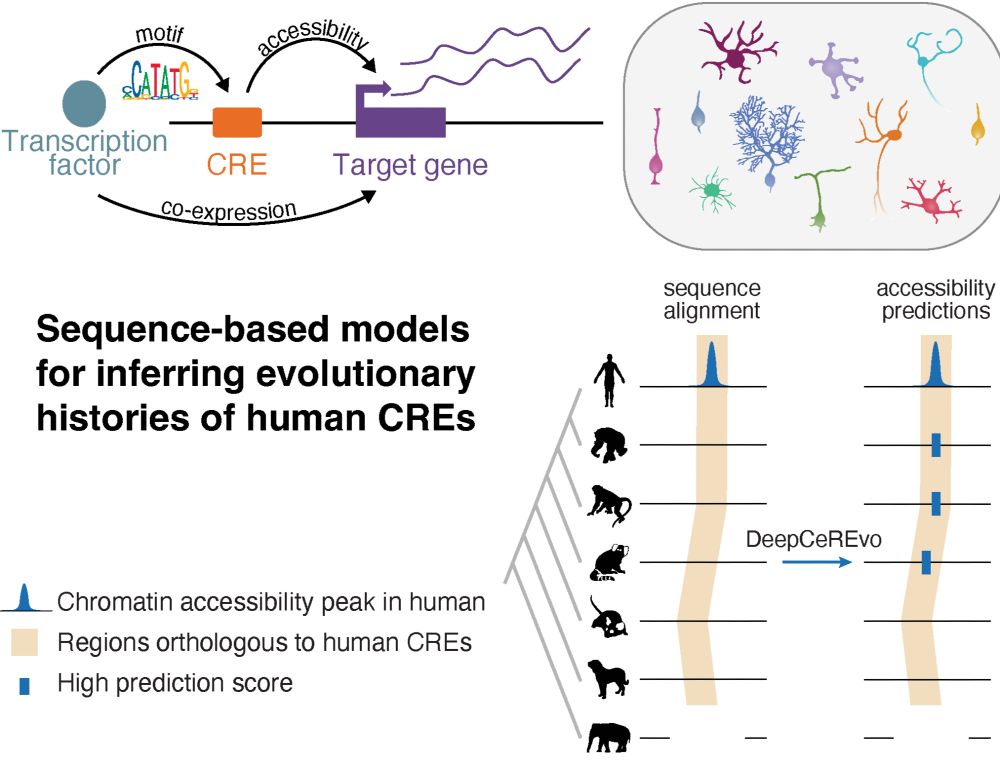
Did transposable elements shape brain evolution — and if so, which ones, and in which cell states and lineages? Led by @tyamadat.bsky.social, we explored this question in cerebellum development using sequence-based deep learning models!
www.biorxiv.org/content/10.1...
www.biorxiv.org/content/10.1...

October 16, 2025 at 10:01 PM
Did transposable elements shape brain evolution — and if so, which ones, and in which cell states and lineages? Led by @tyamadat.bsky.social, we explored this question in cerebellum development using sequence-based deep learning models!
www.biorxiv.org/content/10.1...
www.biorxiv.org/content/10.1...
We are thrilled to share our new preprint entitled “The origin and molecular evolution of the mammalian liver cell architecture” www.biorxiv.org/content/10.1...

October 15, 2025 at 12:51 PM
We are thrilled to share our new preprint entitled “The origin and molecular evolution of the mammalian liver cell architecture” www.biorxiv.org/content/10.1...
👇 !!!
Register to attend the #LouisJeantetSymposium on vertebrate genome #evolution. Organised by Svante Pääbo and
@kaessmannlab.bsky.social . Free event, full line up of speakers 👉 www.jeantet.ch/en/
📆Tuesday 14 October, 08h15
📍CMU - Auditoire Alex-F. Müller (A250)
@genevunige.bsky.social
@kaessmannlab.bsky.social . Free event, full line up of speakers 👉 www.jeantet.ch/en/
📆Tuesday 14 October, 08h15
📍CMU - Auditoire Alex-F. Müller (A250)
@genevunige.bsky.social

September 11, 2025 at 1:09 PM
👇 !!!
Reposted by Kaessmann Lab
🧠🦈Excited to present our latest work🧠🦈Interested in brain evolution? And shark embryos? Then read on… Our work sheds light on the deep origins of our brain’s most complex regions.
September 2, 2025 at 5:51 PM
🧠🦈Excited to present our latest work🧠🦈Interested in brain evolution? And shark embryos? Then read on… Our work sheds light on the deep origins of our brain’s most complex regions.
Reposted by Kaessmann Lab
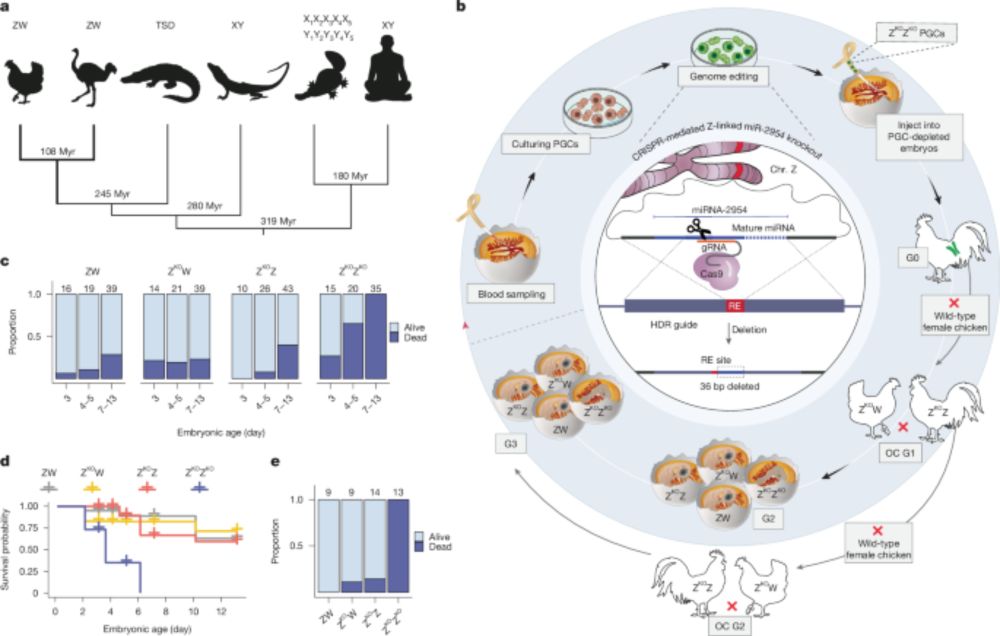
@kaessmannlab.bsky.social has for the first time shown in an international collaboration, that survival of male birds crucially depends on a micro RNA for sex chromosome dosage compensation. The paper just has been published by Nature.
www.nature.com/articles/s41...
www.nature.com/articles/s41...
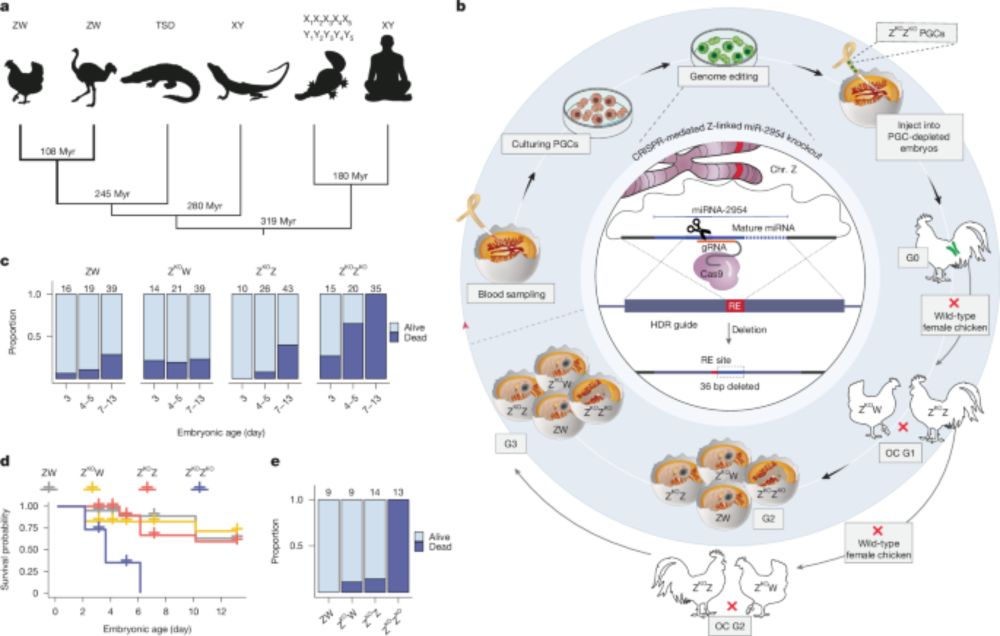
A male-essential miRNA is key for avian sex chromosome dosage compensation - Nature
Birds have evolved a unique sex chromosome dosage compensation mechanism involving the male-biased microRNA (miR-2954), which is essential for male survival by regulating the expression of dosage-sens...
www.nature.com
July 17, 2025 at 8:42 AM
@kaessmannlab.bsky.social has for the first time shown in an international collaboration, that survival of male birds crucially depends on a micro RNA for sex chromosome dosage compensation. The paper just has been published by Nature.
www.nature.com/articles/s41...
www.nature.com/articles/s41...
Reposted by Kaessmann Lab
How a Tiny Gene Ensures the Survival of Male Birds – Researchers from Heidelberg and Edinburgh identify a mechanism that balances the genetic disparity between sex chromosomes
www.uni-heidelberg.de/en/newsroom/...
www.uni-heidelberg.de/en/newsroom/...
July 16, 2025 at 3:34 PM
How a Tiny Gene Ensures the Survival of Male Birds – Researchers from Heidelberg and Edinburgh identify a mechanism that balances the genetic disparity between sex chromosomes
www.uni-heidelberg.de/en/newsroom/...
www.uni-heidelberg.de/en/newsroom/...
Our study on a male-essential microRNA and the evolution of other dosage compensation mechanisms in birds is now out in Nature! www.nature.com/articles/s41...
A male-essential miRNA is key for avian sex chromosome dosage compensation - Nature
Birds have evolved a unique sex chromosome dosage compensation mechanism involving the male-biased microRNA (miR-2954), which is essential for male survival by regulating the expression of dosage-sens...
www.nature.com
July 16, 2025 at 3:23 PM
Our study on a male-essential microRNA and the evolution of other dosage compensation mechanisms in birds is now out in Nature! www.nature.com/articles/s41...
Great work from our wonderful new lab member Anamaria and colleagues in Arnau's lab!
I am very happy to have posted my first bioRxiv preprint. A long time in the making - and still adding a few final touches to it - but we're excited to finally have it out there in the wild:
www.biorxiv.org/content/10.1...
Read below for a few highlights...
www.biorxiv.org/content/10.1...
Read below for a few highlights...
Decoding cnidarian cell type gene regulation
Animal cell types are defined by differential access to genomic information, a process orchestrated by the combinatorial activity of transcription factors that bind to cis -regulatory elements (CREs) to control gene expression. However, the regulatory logic and specific gene networks that define cell identities remain poorly resolved across the animal tree of life. As early-branching metazoans, cnidarians can offer insights into the early evolution of cell type-specific genome regulation. Here, we profiled chromatin accessibility in 60,000 cells from whole adults and gastrula-stage embryos of the sea anemone Nematostella vectensis. We identified 112,728 CREs and quantified their activity across cell types, revealing pervasive combinatorial enhancer usage and distinct promoter architectures. To decode the underlying regulatory grammar, we trained sequence-based models predicting CRE accessibility and used these models to infer ontogenetic relationships among cell types. By integrating sequence motifs, transcription factor expression, and CRE accessibility, we systematically reconstructed the gene regulatory networks that define cnidarian cell types. Our results reveal the regulatory complexity underlying cell differentiation in a morphologically simple animal and highlight conserved principles in animal gene regulation. This work provides a foundation for comparative regulatory genomics to understand the evolutionary emergence of animal cell type diversity. ### Competing Interest Statement The authors have declared no competing interest. European Research Council, https://ror.org/0472cxd90, ERC-StG 851647 Ministerio de Ciencia e Innovación, https://ror.org/05r0vyz12, PID2021-124757NB-I00, FPI Severo Ochoa PhD fellowship European Union, https://ror.org/019w4f821, Marie Skłodowska-Curie INTREPiD co-fund agreement 75442, Marie Skłodowska-Curie grant agreement 101031767
www.biorxiv.org
July 7, 2025 at 3:19 PM
Great work from our wonderful new lab member Anamaria and colleagues in Arnau's lab!
Reposted by Kaessmann Lab
I am very happy to have posted my first bioRxiv preprint. A long time in the making - and still adding a few final touches to it - but we're excited to finally have it out there in the wild:
www.biorxiv.org/content/10.1...
Read below for a few highlights...
www.biorxiv.org/content/10.1...
Read below for a few highlights...
Decoding cnidarian cell type gene regulation
Animal cell types are defined by differential access to genomic information, a process orchestrated by the combinatorial activity of transcription factors that bind to cis -regulatory elements (CREs) to control gene expression. However, the regulatory logic and specific gene networks that define cell identities remain poorly resolved across the animal tree of life. As early-branching metazoans, cnidarians can offer insights into the early evolution of cell type-specific genome regulation. Here, we profiled chromatin accessibility in 60,000 cells from whole adults and gastrula-stage embryos of the sea anemone Nematostella vectensis. We identified 112,728 CREs and quantified their activity across cell types, revealing pervasive combinatorial enhancer usage and distinct promoter architectures. To decode the underlying regulatory grammar, we trained sequence-based models predicting CRE accessibility and used these models to infer ontogenetic relationships among cell types. By integrating sequence motifs, transcription factor expression, and CRE accessibility, we systematically reconstructed the gene regulatory networks that define cnidarian cell types. Our results reveal the regulatory complexity underlying cell differentiation in a morphologically simple animal and highlight conserved principles in animal gene regulation. This work provides a foundation for comparative regulatory genomics to understand the evolutionary emergence of animal cell type diversity. ### Competing Interest Statement The authors have declared no competing interest. European Research Council, https://ror.org/0472cxd90, ERC-StG 851647 Ministerio de Ciencia e Innovación, https://ror.org/05r0vyz12, PID2021-124757NB-I00, FPI Severo Ochoa PhD fellowship European Union, https://ror.org/019w4f821, Marie Skłodowska-Curie INTREPiD co-fund agreement 75442, Marie Skłodowska-Curie grant agreement 101031767
www.biorxiv.org
July 6, 2025 at 6:15 PM
I am very happy to have posted my first bioRxiv preprint. A long time in the making - and still adding a few final touches to it - but we're excited to finally have it out there in the wild:
www.biorxiv.org/content/10.1...
Read below for a few highlights...
www.biorxiv.org/content/10.1...
Read below for a few highlights...
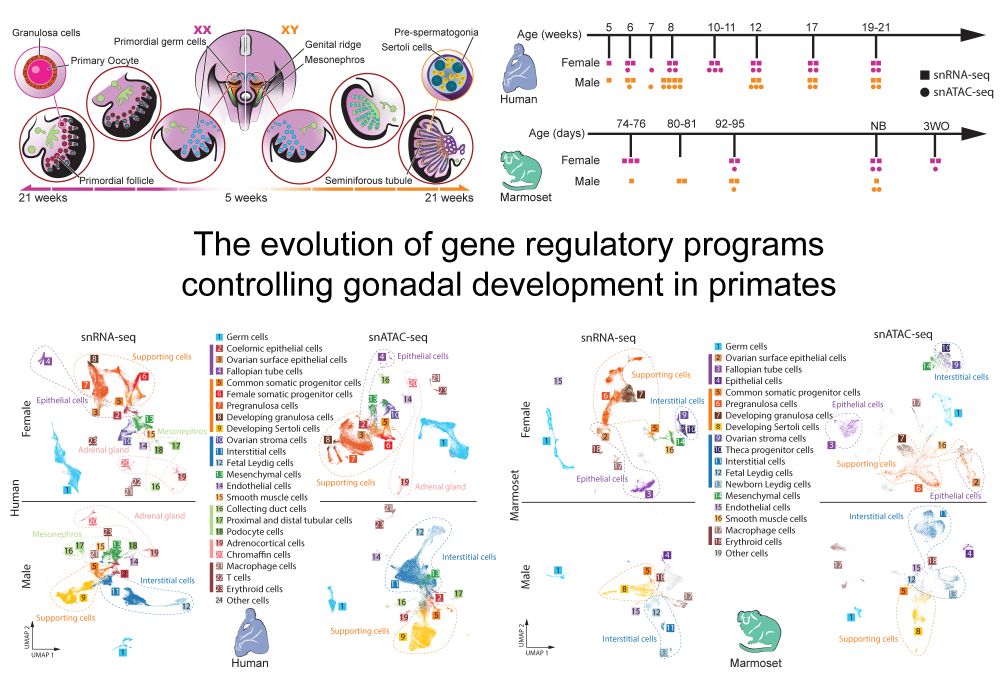
We are delighted to share our new preprint “The evolution of gene regulatory programs controlling gonadal development in primates” www.biorxiv.org/content/10.1...
June 20, 2025 at 8:13 AM
We are delighted to share our new preprint “The evolution of gene regulatory programs controlling gonadal development in primates” www.biorxiv.org/content/10.1...
A great article highlighting the broader implications of the three parallel recent papers from our group and our wonderful colleagues in Spain and Belgium...
Calling someone bird-brained is, in fact, a way of calling someone highly intelligent. @yaseminsaplakoglu.bsky.social reports: www.quantamagazine.org/intelligence...

Intelligence Evolved at Least Twice in Vertebrate Animals | Quanta Magazine
Complex neural circuits likely arose independently in birds and mammals, suggesting that vertebrates evolved intelligence multiple times.
www.quantamagazine.org
April 7, 2025 at 3:49 PM
A great article highlighting the broader implications of the three parallel recent papers from our group and our wonderful colleagues in Spain and Belgium...
Reposted by Kaessmann Lab
Fantastic study and collaboration led by stellar postdoc @ioansarr.bsky.social, it was a pleasure to host you in the lab (& thanks to @embo.org for the visiting fellowship)
How does gene regulation shape brain evolution? Our new preprint dives into this question in the context of mammalian cerebellum development! rb.gy/dbcxjz
Led by @ioansarr.bsky.social, @marisepp.bsky.social and @tyamadat.bsky.social, in collaboration with @steinaerts.bsky.social
Led by @ioansarr.bsky.social, @marisepp.bsky.social and @tyamadat.bsky.social, in collaboration with @steinaerts.bsky.social
March 16, 2025 at 12:55 PM
Fantastic study and collaboration led by stellar postdoc @ioansarr.bsky.social, it was a pleasure to host you in the lab (& thanks to @embo.org for the visiting fellowship)
How does gene regulation shape brain evolution? Our new preprint dives into this question in the context of mammalian cerebellum development! rb.gy/dbcxjz
Led by @ioansarr.bsky.social, @marisepp.bsky.social and @tyamadat.bsky.social, in collaboration with @steinaerts.bsky.social
Led by @ioansarr.bsky.social, @marisepp.bsky.social and @tyamadat.bsky.social, in collaboration with @steinaerts.bsky.social

March 16, 2025 at 10:31 AM
How does gene regulation shape brain evolution? Our new preprint dives into this question in the context of mammalian cerebellum development! rb.gy/dbcxjz
Led by @ioansarr.bsky.social, @marisepp.bsky.social and @tyamadat.bsky.social, in collaboration with @steinaerts.bsky.social
Led by @ioansarr.bsky.social, @marisepp.bsky.social and @tyamadat.bsky.social, in collaboration with @steinaerts.bsky.social
Reposted by Kaessmann Lab
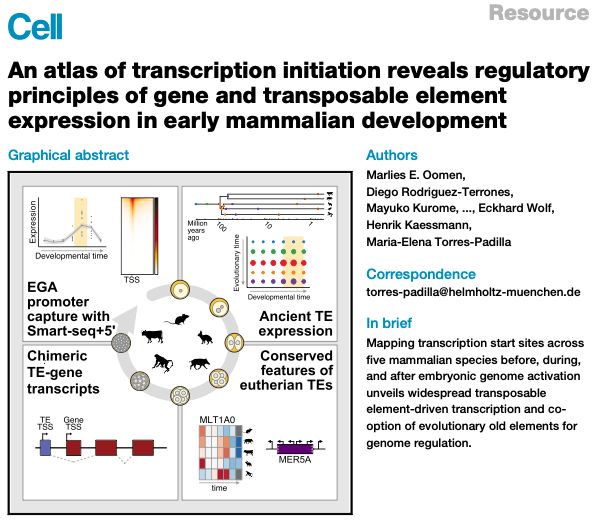
Paper out !!🥳big thanks to all authors @marliesoomen.bsky.social @diego-rt.bsky.social @kaessmannlab.bsky.social @jonathangoeke.bsky.social @lorenzamottes.bsky.social & 'bluesky-less'
Lots of interesting new TE (& genes) biology
Data fully browsable💻 👉 embryo.helmholtz-munich.de/shiny_embryo/
Lots of interesting new TE (& genes) biology
Data fully browsable💻 👉 embryo.helmholtz-munich.de/shiny_embryo/
February 21, 2025 at 3:50 PM
Paper out !!🥳big thanks to all authors @marliesoomen.bsky.social @diego-rt.bsky.social @kaessmannlab.bsky.social @jonathangoeke.bsky.social @lorenzamottes.bsky.social & 'bluesky-less'
Lots of interesting new TE (& genes) biology
Data fully browsable💻 👉 embryo.helmholtz-munich.de/shiny_embryo/
Lots of interesting new TE (& genes) biology
Data fully browsable💻 👉 embryo.helmholtz-munich.de/shiny_embryo/
Great colleague/lab and great opportunity!
Hi everyone! We are still welcoming applicants for a computational postdoc position. If your profile fits our lab interests, please do reach out to me for more information or apply directly via the link below. I'm looking forward to reading your application! www.academictransfer.com/en/jobs/3488...
February 18, 2025 at 12:28 PM
Great colleague/lab and great opportunity!
Reposted by Kaessmann Lab
Hi everyone! We are still welcoming applicants for a computational postdoc position. If your profile fits our lab interests, please do reach out to me for more information or apply directly via the link below. I'm looking forward to reading your application! www.academictransfer.com/en/jobs/3488...
February 18, 2025 at 12:00 PM
Hi everyone! We are still welcoming applicants for a computational postdoc position. If your profile fits our lab interests, please do reach out to me for more information or apply directly via the link below. I'm looking forward to reading your application! www.academictransfer.com/en/jobs/3488...
Reposted by Kaessmann Lab
Two days after our preprint in the role of active DNA methylation in retinal development... Happy to share our last paper from my former lab. SnRNA-seq atlas of the postnatal shark retina!! www.nature.com/articles/s41...
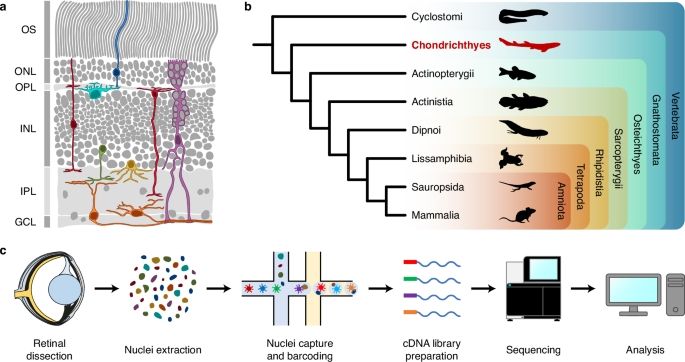
A single-nucleus RNA sequencing atlas of the postnatal retina of the shark Scyliorhinus canicula - Scientific Data
Scientific Data - A single-nucleus RNA sequencing atlas of the postnatal retina of the shark Scyliorhinus canicula
www.nature.com
February 7, 2025 at 3:18 PM
Two days after our preprint in the role of active DNA methylation in retinal development... Happy to share our last paper from my former lab. SnRNA-seq atlas of the postnatal shark retina!! www.nature.com/articles/s41...
Reposted by Kaessmann Lab
A big cheer for Bassi @bassi-z.bsky.social, the newest doctor in the @kaessmannlab.bsky.social! An absolute inspiration!
What a week! I defended my PhD on Monday, and now my first first-author paper was published in @science.org.
shorturl.at/fvIGZ
I am so incredibly grateful to everyone who made this possible! Especially @kaessmannlab.bsky.social and the García-Moreno lab 💛
shorturl.at/fvIGZ
I am so incredibly grateful to everyone who made this possible! Especially @kaessmannlab.bsky.social and the García-Moreno lab 💛


February 14, 2025 at 7:12 PM
A big cheer for Bassi @bassi-z.bsky.social, the newest doctor in the @kaessmannlab.bsky.social! An absolute inspiration!
Reposted by Kaessmann Lab
What a week! I defended my PhD on Monday, and now my first first-author paper was published in @science.org.
shorturl.at/fvIGZ
I am so incredibly grateful to everyone who made this possible! Especially @kaessmannlab.bsky.social and the García-Moreno lab 💛
shorturl.at/fvIGZ
I am so incredibly grateful to everyone who made this possible! Especially @kaessmannlab.bsky.social and the García-Moreno lab 💛


February 14, 2025 at 6:27 PM
What a week! I defended my PhD on Monday, and now my first first-author paper was published in @science.org.
shorturl.at/fvIGZ
I am so incredibly grateful to everyone who made this possible! Especially @kaessmannlab.bsky.social and the García-Moreno lab 💛
shorturl.at/fvIGZ
I am so incredibly grateful to everyone who made this possible! Especially @kaessmannlab.bsky.social and the García-Moreno lab 💛
Reposted by Kaessmann Lab
Komplexe Evolution: Fortgeschrittene kognitive Fähigkeiten bei Vögeln – Heidelberger Forscher kartieren verantwortliche Gehirnregionen und gewinnen neue Erkenntnisse zu ihrer embryonalen und evolutionären Entwicklung www.uni-heidelberg.de/de/newsroom/...

February 14, 2025 at 8:49 AM
Komplexe Evolution: Fortgeschrittene kognitive Fähigkeiten bei Vögeln – Heidelberger Forscher kartieren verantwortliche Gehirnregionen und gewinnen neue Erkenntnisse zu ihrer embryonalen und evolutionären Entwicklung www.uni-heidelberg.de/de/newsroom/...
Reposted by Kaessmann Lab
.. and what an honour to be jointly published with two other papers from the @kaessmannlab.bsky.social and the Fernando García-Moreno lab - all of this summarized in a great perspective by Maria Tosches and @giacomogattoni.bsky.social www.science.org/doi/10.1126/...
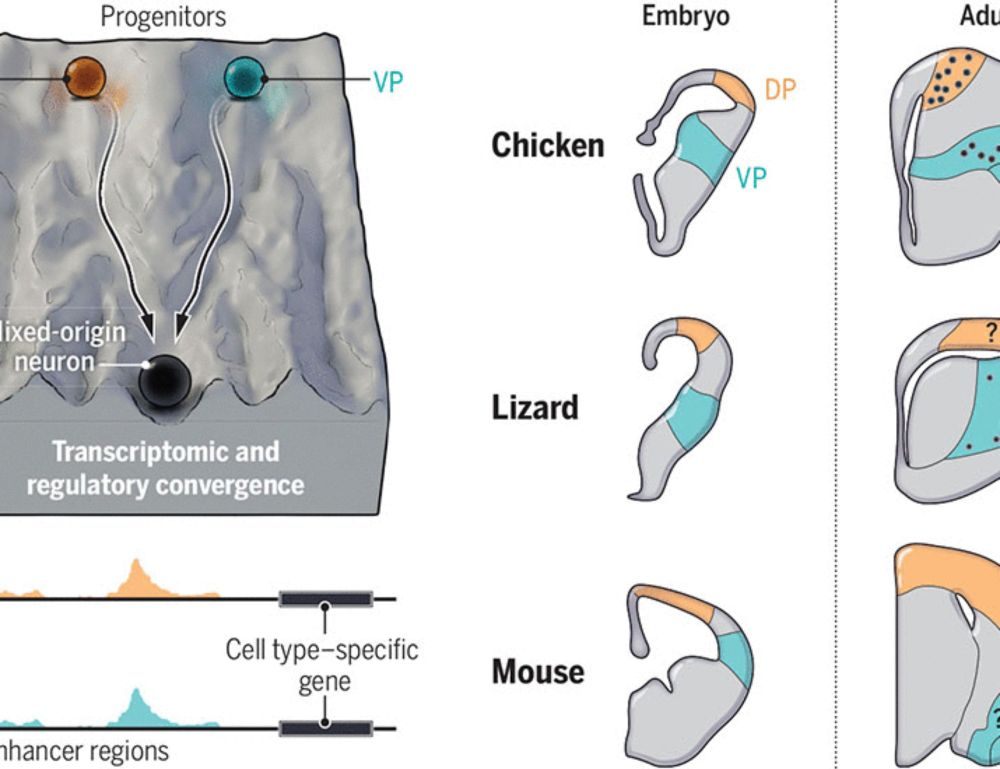
Constrained roads to complex brains
Neural development and brain circuit evolution converged in birds and mammals
www.science.org
February 14, 2025 at 12:06 PM
.. and what an honour to be jointly published with two other papers from the @kaessmannlab.bsky.social and the Fernando García-Moreno lab - all of this summarized in a great perspective by Maria Tosches and @giacomogattoni.bsky.social www.science.org/doi/10.1126/...
Reposted by Kaessmann Lab
#CellPlasticity—the ability of cells to change their identity—is vital for tissue growth and repair. But when it goes unchecked, it can fuel #cancer. Our latest study examines how to block #LiverCancer by actively suppressing plasticity. www.nature.com/articles/s41... #CancerBiology

February 13, 2025 at 12:16 PM
#CellPlasticity—the ability of cells to change their identity—is vital for tissue growth and repair. But when it goes unchecked, it can fuel #cancer. Our latest study examines how to block #LiverCancer by actively suppressing plasticity. www.nature.com/articles/s41... #CancerBiology
Reposted by Kaessmann Lab
Three fantastic @science.org papers on convergent development & evolution of neurons + their connections in the bird &
mammalian pallia, by @kaessmannlab.bsky.social @steinaerts.bsky.social & Fernando García-Moreno.
Expertly synthesised by @giacomogattoni.bsky.social & Maria Antonietta Tosches 🧪🧠
mammalian pallia, by @kaessmannlab.bsky.social @steinaerts.bsky.social & Fernando García-Moreno.
Expertly synthesised by @giacomogattoni.bsky.social & Maria Antonietta Tosches 🧪🧠

Constrained roads to complex brains
Neural development and brain circuit evolution converged in birds and mammals
www.science.org
February 14, 2025 at 6:52 AM
Three fantastic @science.org papers on convergent development & evolution of neurons + their connections in the bird &
mammalian pallia, by @kaessmannlab.bsky.social @steinaerts.bsky.social & Fernando García-Moreno.
Expertly synthesised by @giacomogattoni.bsky.social & Maria Antonietta Tosches 🧪🧠
mammalian pallia, by @kaessmannlab.bsky.social @steinaerts.bsky.social & Fernando García-Moreno.
Expertly synthesised by @giacomogattoni.bsky.social & Maria Antonietta Tosches 🧪🧠

