





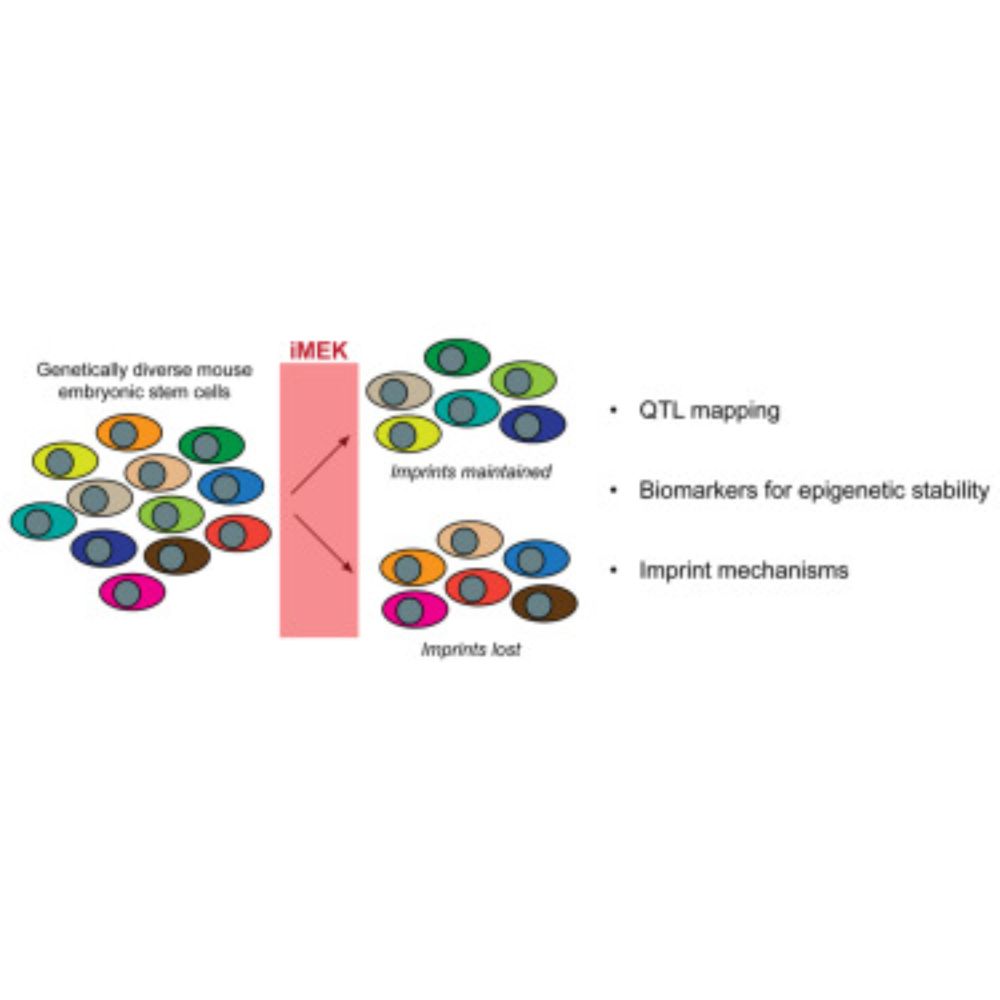
👉🏿https://rdcu.be/eayN3
www.nature.com/articles/s41...

👉🏿https://rdcu.be/eayN3
www.nature.com/articles/s41...
rdcu.be/d8awu
www.nature.com/articles/s41...
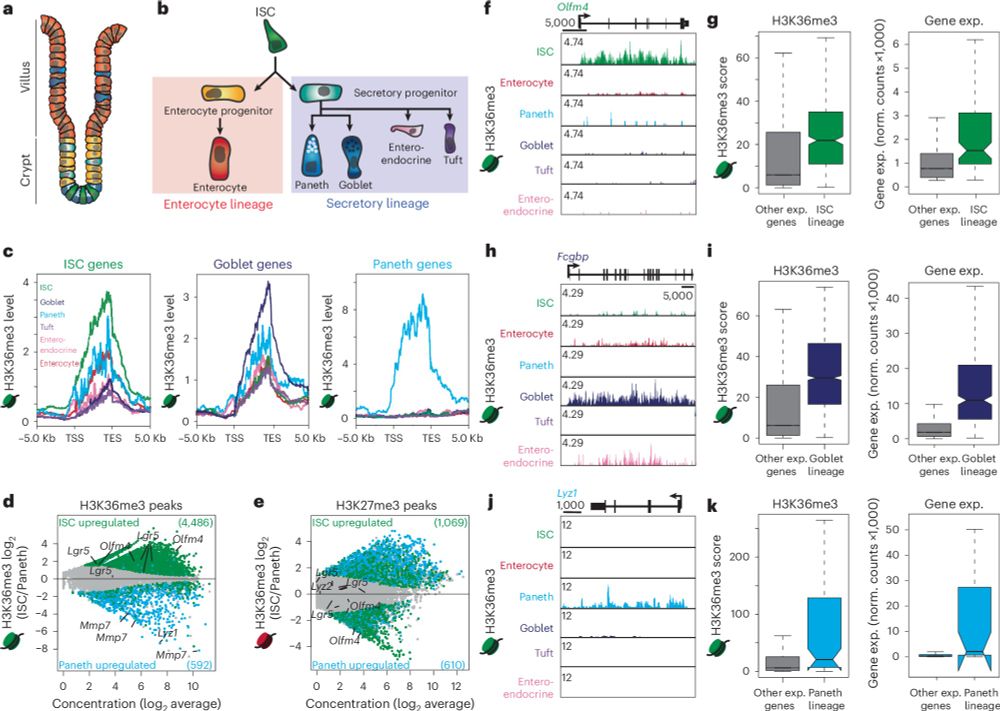
rdcu.be/d8awu
www.nature.com/articles/s41...
@natrevimmunol.bsky.social, highlighting how biomolecular condensates enhance the precision and flexibility of gene regulatory networks that guide fate decisions during normal and pathological immune cell development. www.nature.com/articles/s41...

@natrevimmunol.bsky.social, highlighting how biomolecular condensates enhance the precision and flexibility of gene regulatory networks that guide fate decisions during normal and pathological immune cell development. www.nature.com/articles/s41...
Thanks to our entire team for their tireless work and to the editors at @naturecellbiology.bsky.social for the opportunity!
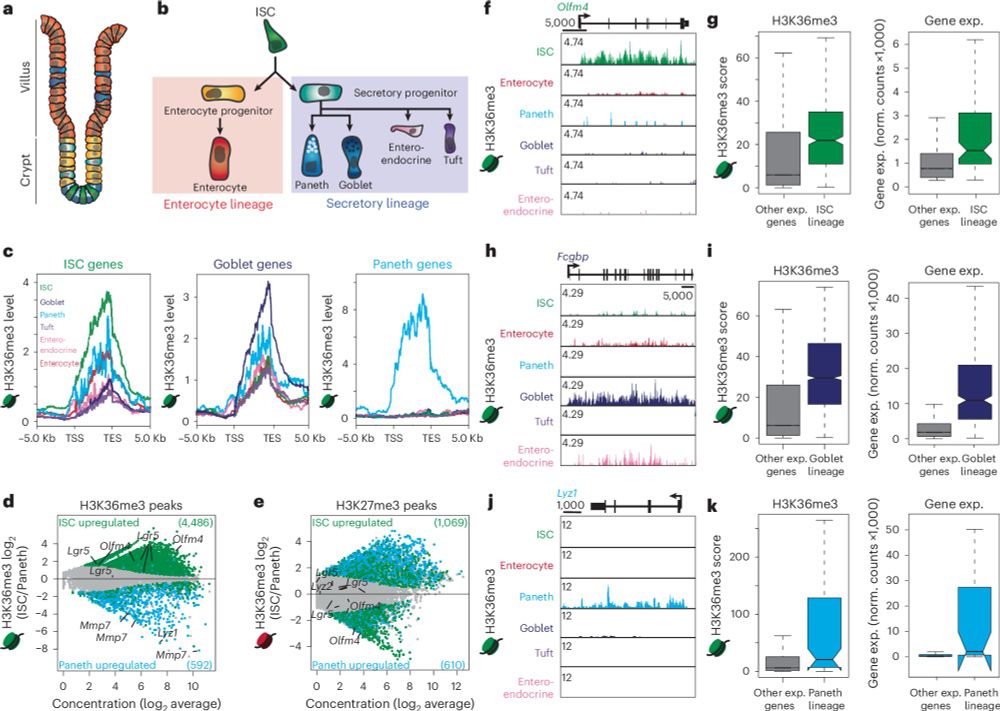
Thanks to our entire team for their tireless work and to the editors at @naturecellbiology.bsky.social for the opportunity!

