Article by Tehshik P. Yoon & co-workers @tehshik.bsky.social @uwyoongroup.bsky.social
Oxygen migration into carbon–carbon single bonds by photochemical oxidation
www.nature.com/articles/s44...
#Chemsky

Article by Tehshik P. Yoon & co-workers @tehshik.bsky.social @uwyoongroup.bsky.social
Oxygen migration into carbon–carbon single bonds by photochemical oxidation
www.nature.com/articles/s44...
#Chemsky
I could write a whole article about this, but as one example:
“To close observers, the original crisis began well before any of this…”
No. I’m a close observer of science, and this is incorrect.
I could write a whole article about this, but as one example:
“To close observers, the original crisis began well before any of this…”
No. I’m a close observer of science, and this is incorrect.
chemsky 🧪
www.science.org/doi/10.1126/...

chemsky 🧪
www.science.org/doi/10.1126/...

New reactions are typically developed by trial and error. How can we speed up this process? Read on to learn how we used DNA scaffolding to perform >500,000 parallel reactions on attomole scale.
1/n
New reactions are typically developed by trial and error. How can we speed up this process? Read on to learn how we used DNA scaffolding to perform >500,000 parallel reactions on attomole scale.
1/n
The award is sponsored by @OrgReactions and @OrgSynth
Congratulations!


The award is sponsored by @OrgReactions and @OrgSynth
Congratulations!
Deadline: July 6
Link to our recent work in comments.

Deadline: July 6
Link to our recent work in comments.
Reposts appreciated!
jobs.ethz.ch/job/view/JOP...

Reposts appreciated!
jobs.ethz.ch/job/view/JOP...
www.queensu.ca/grad-postdoc...
www.queensu.ca/grad-postdoc...
Grad programs are re-opening applications of US programs for one week. With expedited decisions.
U.S. Applicant Week:
www.grad.ubc.ca/us-applicant...
#AcademicChatter #Canada #GradSchool
Grad programs are re-opening applications of US programs for one week. With expedited decisions.
U.S. Applicant Week:
www.grad.ubc.ca/us-applicant...
#AcademicChatter #Canada #GradSchool
pubs.acs.org/doi/full/10....
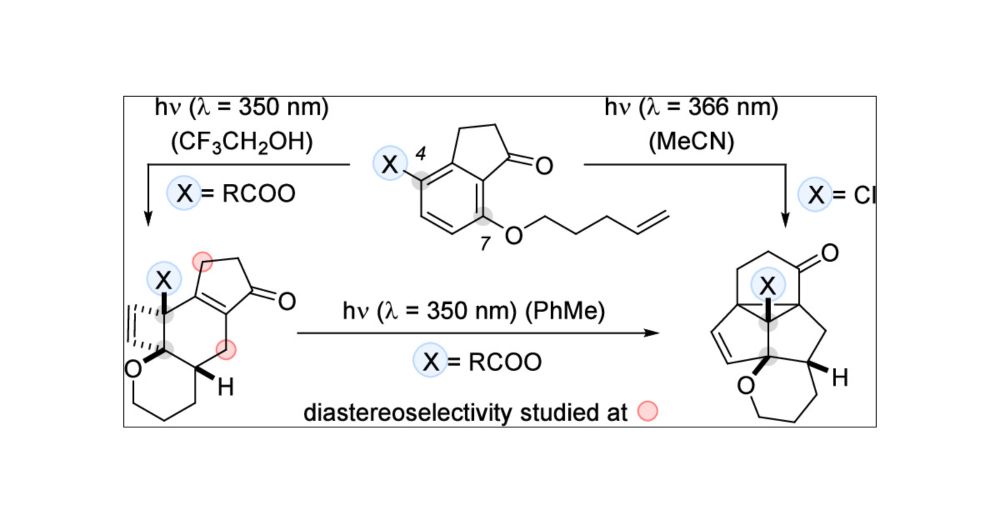
pubs.acs.org/doi/full/10....
pubs.acs.org/doi/10.1021/...
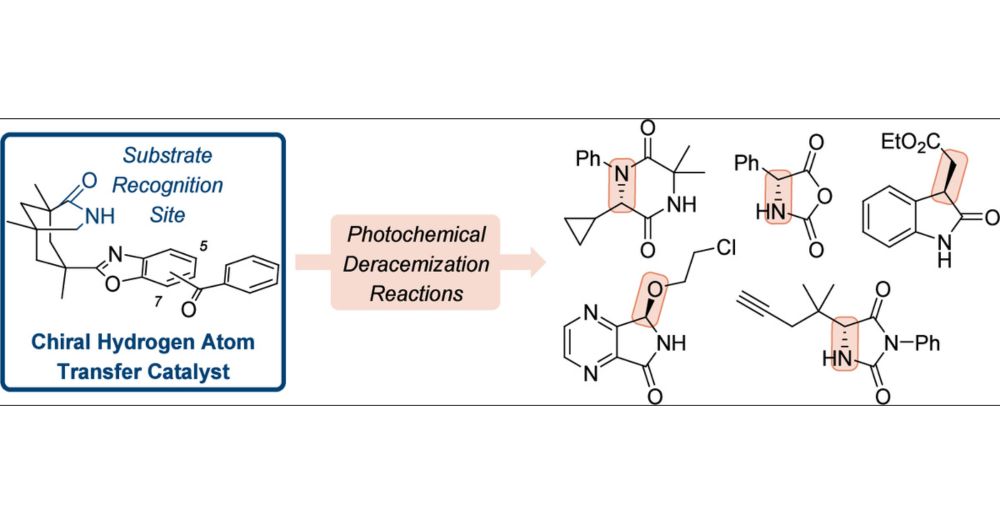
pubs.acs.org/doi/10.1021/...
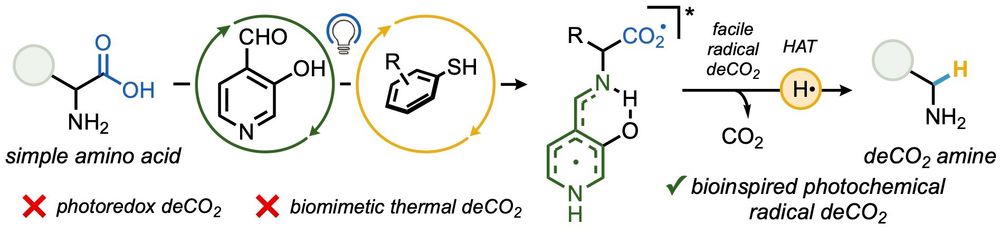

Photochem-mediated oxygen migration into sp3 bonds delivering small ring ethers as well as an OH - methylene transposition
#ChemSky 1/
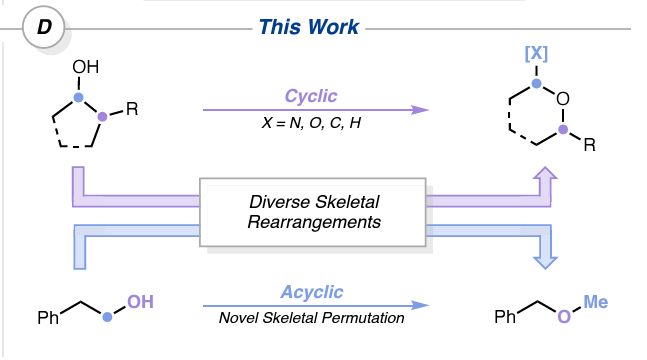
Photochem-mediated oxygen migration into sp3 bonds delivering small ring ethers as well as an OH - methylene transposition
#ChemSky 1/
chemrxiv.org/engage/chemr...
chemrxiv.org/engage/chemr...

chemrxiv.org/engage/chemr...
chemrxiv.org/engage/chemr...

We are seeking a new Chief Editor for @naturesynthesis.bsky.social 🧪
📍 Beijing, New York or Shanghai
🗓️ Closing date: Dec 31, 2024
✉️ DMs open for questions
🙏 Re-posts very much appreciated!
springernature.wd3.myworkdayjobs.com/SpringerNatu...
#Chemsky #Editorial #Publishing