Harrogate, TN
https://pubmed.ncbi.nlm.nih.gov/?term=kathayat+D&sort=date
We discuss how tethering the OM to the PG in E. coli preserves integrity — and extend the concept across diderm bacteria.
Curr Opin Microbiol: doi.org/10.1016/j.mi...
#microsky 🔬
We discuss how tethering the OM to the PG in E. coli preserves integrity — and extend the concept across diderm bacteria.
Curr Opin Microbiol: doi.org/10.1016/j.mi...
#microsky 🔬


A great collab. w/ @mygalperin.bsky.social @vikramalva.bsky.social @thethormannden.bsky.social
www.pnas.org/doi/10.1073/...
@hhu.de @cmfi.bsky.social
@sfb1381.bsky.social
@mibinet.bsky.social

A great collab. w/ @mygalperin.bsky.social @vikramalva.bsky.social @thethormannden.bsky.social
www.pnas.org/doi/10.1073/...
@hhu.de @cmfi.bsky.social
@sfb1381.bsky.social
@mibinet.bsky.social

journals.asm.org/doi/10.1128/...

journals.asm.org/doi/10.1128/...
We challenge the long-standing view that peptidoglycan alone protects cells from bursting.
Our study shows that the periplasm — enclosed by OM–PG connections — acts as a pressure buffer essential for osmoprotection in Gram-negative bacteria.
📄 www.nature.com/articles/s41...
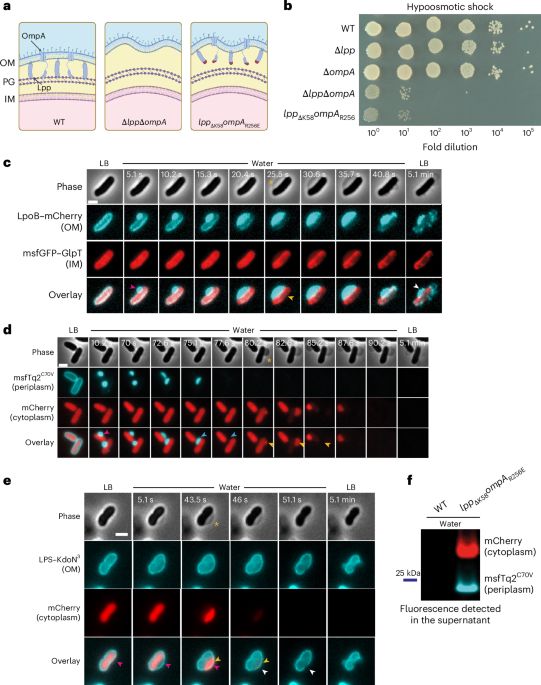
We challenge the long-standing view that peptidoglycan alone protects cells from bursting.
Our study shows that the periplasm — enclosed by OM–PG connections — acts as a pressure buffer essential for osmoprotection in Gram-negative bacteria.
📄 www.nature.com/articles/s41...
journals.asm.org/doi/10.1128/...

journals.asm.org/doi/10.1128/...
https://go.nature.com/3XmyOPD
https://go.nature.com/3XmyOPD
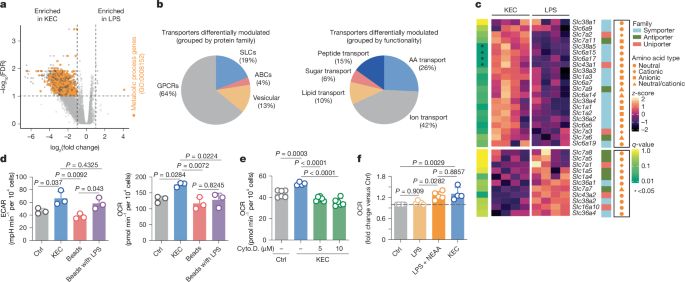
journals.asm.org/toc/jb/207/2
@asm.org #JBacteriology

journals.asm.org/toc/jb/207/2
@asm.org #JBacteriology

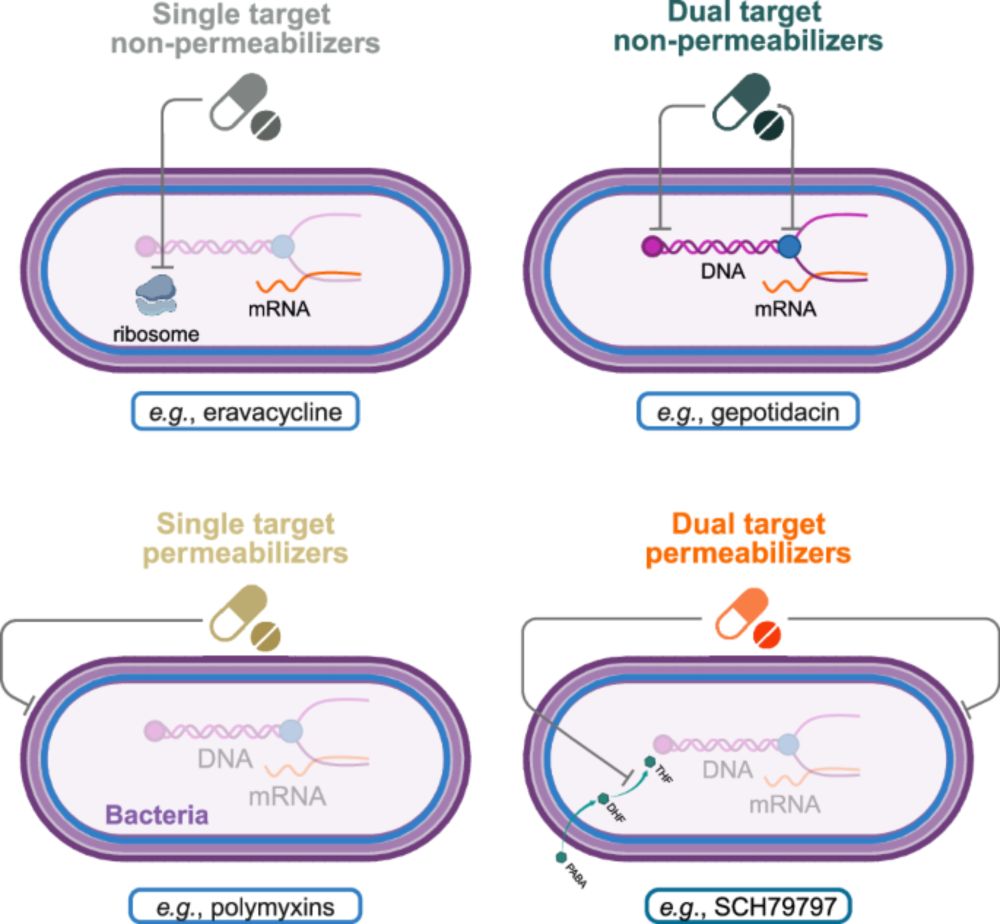
journals.asm.org/doi/10.1128/...

journals.asm.org/doi/10.1128/...

