
doi.org/10.26434/che...

doi.org/10.26434/che...

Join our group in Bioinorganic Chemistry to work on the synthesis & biological evaluation of metal complexes for anticancer therapy.
jobs.ruhr-uni-bochum.de/jobposting/8...


Join our group in Bioinorganic Chemistry to work on the synthesis & biological evaluation of metal complexes for anticancer therapy.
jobs.ruhr-uni-bochum.de/jobposting/8...

pubs.acs.org/doi/full/10....
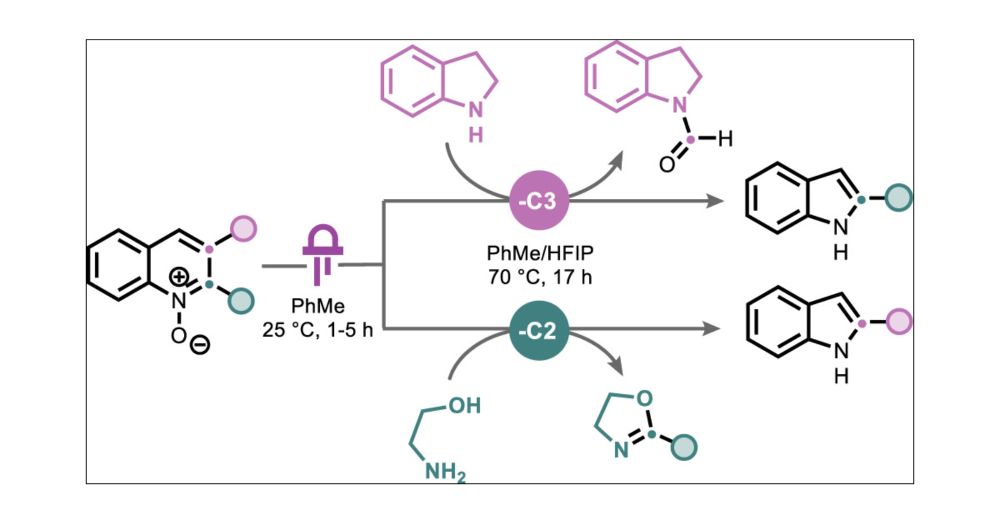
pubs.acs.org/doi/full/10....
pubs.acs.org/doi/10.1021/...
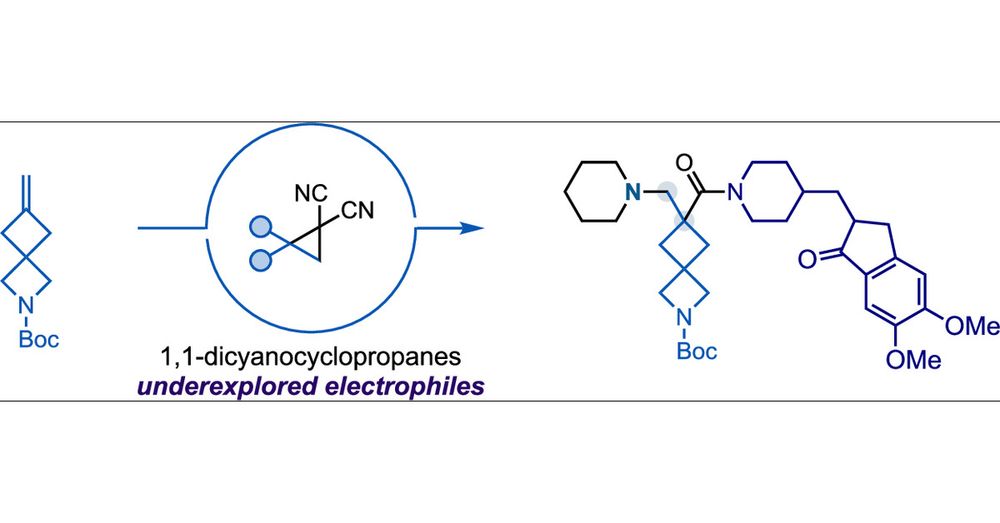
pubs.acs.org/doi/10.1021/...
pubs.acs.org/doi/10.1021/...

pubs.acs.org/doi/10.1021/...
We have an exciting opportunity to join our research group!
This fully funded PhD position is part of the IPCat training programme.
Feel free to reach out if you have any questions or need more information.
👉 Apply now: www.findaphd.com/phds/project...
#PhD #compchem
We have an exciting opportunity to join our research group!
This fully funded PhD position is part of the IPCat training programme.
Feel free to reach out if you have any questions or need more information.
👉 Apply now: www.findaphd.com/phds/project...
#PhD #compchem
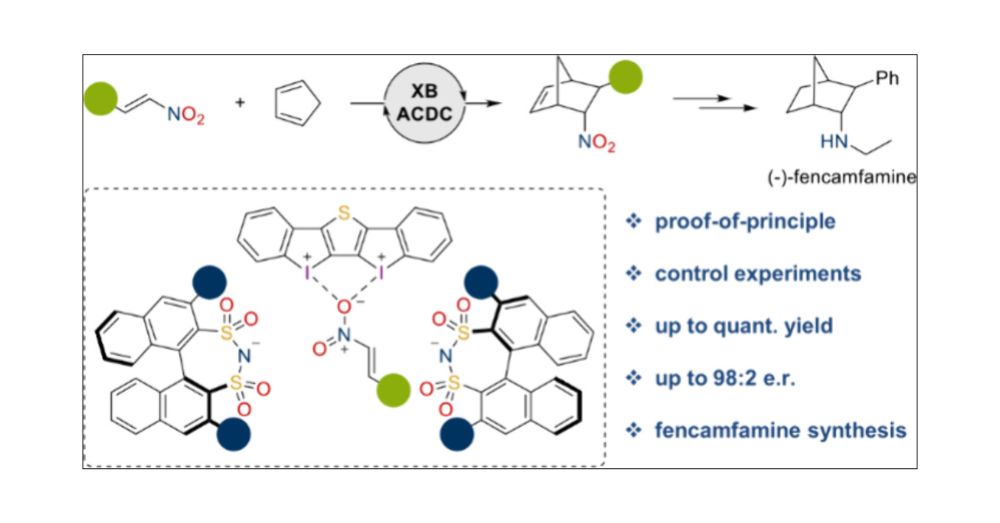
This is the beginning of a long journey for us, to study heterogeneity in GBM patient samples.
First step was to figure out some chemistry to enable us to look at this challenging problem.
pubs.acs.org/doi/10.1021/...

This is the beginning of a long journey for us, to study heterogeneity in GBM patient samples.
First step was to figure out some chemistry to enable us to look at this challenging problem.
pubs.acs.org/doi/10.1021/...
pubs.acs.org/doi/10.1021/...
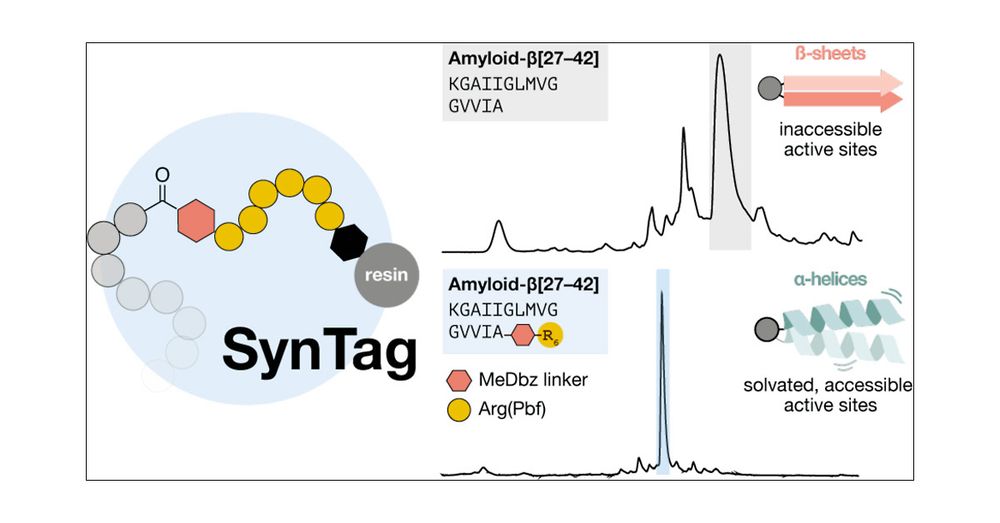
pubs.acs.org/doi/10.1021/...

Given recent successful grant applications (I got my SNSF Starting Grant 🚀), we are extending the LIAC team with multiple openings (PhD/postdoc) for 2025.
Apply now (deadline: December 20th) by filling in this form: forms.fillout.com/t/eq5ADAw3kkus.
#ChemSky



Given recent successful grant applications (I got my SNSF Starting Grant 🚀), we are extending the LIAC team with multiple openings (PhD/postdoc) for 2025.
Apply now (deadline: December 20th) by filling in this form: forms.fillout.com/t/eq5ADAw3kkus.
#ChemSky


