Self-Assembling Peptide Amphiphile 3D models of the Tumour-Immune Microenvironment of Paediatric Posterior Fossa Tumours.
PhD St Andrews, Goss Group.
Postdoc Astbury Centre, Leeds, Riobo-Del Galdo

www.childrenwithcancer.org.uk/childhood-ca...

Precision Molecular Editing: Predicting Substrate Scope and Regiochemistry for CHEESY1, a Flavin Dependent Halogenase | ACS Catalysis pubs.acs.org/doi/10.1021/...

Precision Molecular Editing: Predicting Substrate Scope and Regiochemistry for CHEESY1, a Flavin Dependent Halogenase | ACS Catalysis pubs.acs.org/doi/10.1021/...
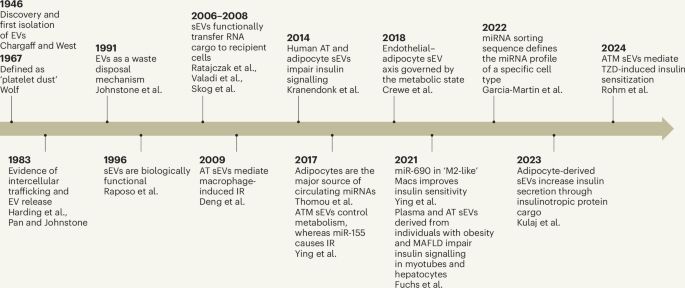
Our newest study just out today!

Our newest study just out today!
www.science.org/doi/10.1126/...
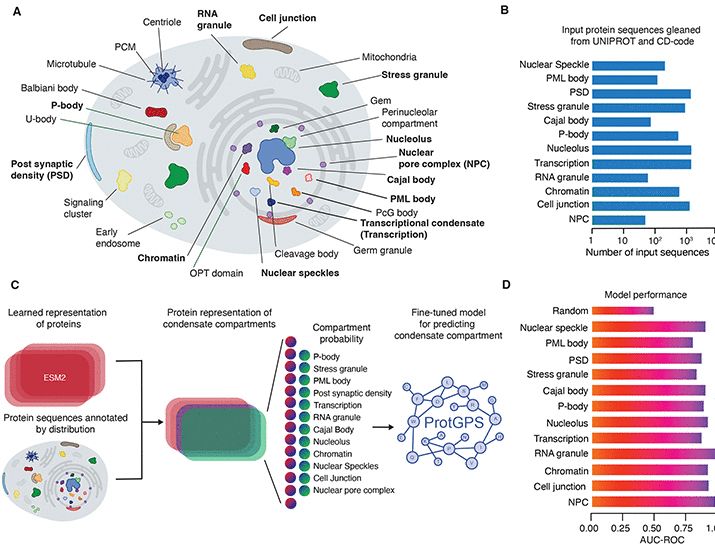
www.science.org/doi/10.1126/...


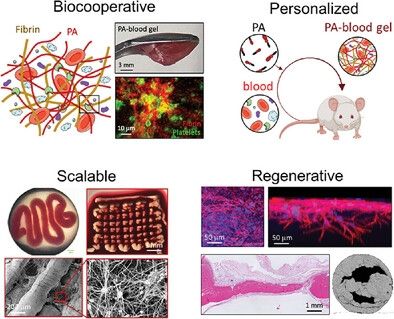
Alvaro Mata, Cosimo Ligorio, Domenico Laurenza
www.nottingham.ac.uk/news/scienti...
Alvaro Mata, Cosimo Ligorio, Domenico Laurenza
www.nottingham.ac.uk/news/scienti...
onlinelibrary.wiley.com/doi/10.1002/...
onlinelibrary.wiley.com/doi/10.1002/...
www.childrenwithcancer.org.uk/childhood-ca...

www.childrenwithcancer.org.uk/childhood-ca...