
Alex Bukvic and Mathis Brändlin in @jacs.acspublications.org
pubs.acs.org/doi/10.1021/...

Alex Bukvic and Mathis Brändlin in @jacs.acspublications.org
pubs.acs.org/doi/10.1021/...
Florian Doettinger, Jonathan Sagaya, Giacomo Morselli in @jacs.acspublications.org
pubs.acs.org/doi/10.1021/...

Florian Doettinger, Jonathan Sagaya, Giacomo Morselli in @jacs.acspublications.org
pubs.acs.org/doi/10.1021/...
tu-braunschweig.webex.com/tu-braunschw...
key: Photo2025
5 talks by talented students (one will be awarded🎉) followed by a plenary lecture. Save the date. ✍️

tu-braunschweig.webex.com/tu-braunschw...
key: Photo2025
5 talks by talented students (one will be awarded🎉) followed by a plenary lecture. Save the date. ✍️
Huge thanks to Bekah for leading the experiments and shaping the story🥳 a true collaborative effort!😃
pubs.acs.org/doi/10.1021/...

Huge thanks to Bekah for leading the experiments and shaping the story🥳 a true collaborative effort!😃
pubs.acs.org/doi/10.1021/...
Mathis Brändlin and Felix Himmelreich in jacsau.bsky.social
pubs.acs.org/doi/full/10....

Mathis Brändlin and Felix Himmelreich in jacsau.bsky.social
pubs.acs.org/doi/full/10....
pubs.rsc.org/en/content/a...

pubs.rsc.org/en/content/a...
This review describes the synthesis, structure–property relationships, and examples of applications for seven important classes of photoswitches
doi.org/10.3762/bjoc...

This review describes the synthesis, structure–property relationships, and examples of applications for seven important classes of photoswitches
doi.org/10.3762/bjoc...
Many thanks to our collaboration partner @nobuhiroyanai.bsky.social and Masanori.

Many thanks to our collaboration partner @nobuhiroyanai.bsky.social and Masanori.

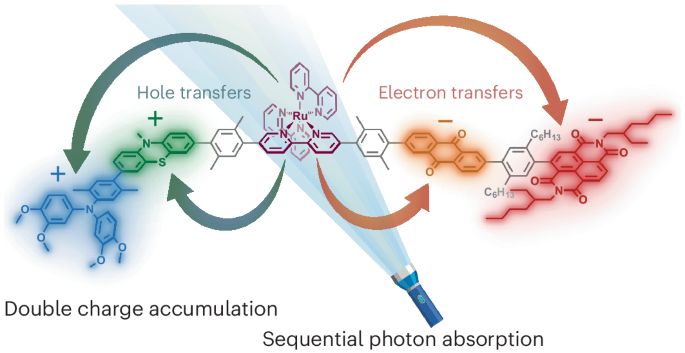
100 ns lifetime, 3 eV energy storage, and 37% quantum yield
A step toward multi-electron photochemistry
Mathis Brändlin and @bjoernpfund.bsky.social in @natchem.nature.com
www.nature.com/articles/s41...

100 ns lifetime, 3 eV energy storage, and 37% quantum yield
A step toward multi-electron photochemistry
Mathis Brändlin and @bjoernpfund.bsky.social in @natchem.nature.com
www.nature.com/articles/s41...
pubs.acs.org/doi/10.1021/...

pubs.acs.org/doi/10.1021/...

Using fsTA, we discovered that charge separation mediates Energy Transfer in the long-lived⏳ iron complex-anthracene dyad designed and synthesized by Felix Glaser & @ludotroian.bsky.social - just published in ACS Central Science @pubs.acs.org 🥳
t.co/14xmp6kvHS
Using fsTA, we discovered that charge separation mediates Energy Transfer in the long-lived⏳ iron complex-anthracene dyad designed and synthesized by Felix Glaser & @ludotroian.bsky.social - just published in ACS Central Science @pubs.acs.org 🥳
t.co/14xmp6kvHS
We show that higher-lying excited states can drive electron transfer — opening new doors for photoredox catalysis.
Huge thanks to @wengeroliver.bsky.social for the incredible mentorship!
pubs.acs.org/doi/10.1021/...
We show that higher-lying excited states can drive electron transfer — opening new doors for photoredox catalysis.
Huge thanks to @wengeroliver.bsky.social for the incredible mentorship!
pubs.acs.org/doi/10.1021/...
For Fe(III) complexes, excited-state redox potentials don’t follow the usual rules - standard estimation methods fall short
Joël Wellauer with Paul Francis & colleagues at Deakin University in @jacs.acspublications.org
pubs.acs.org/doi/10.1021/...
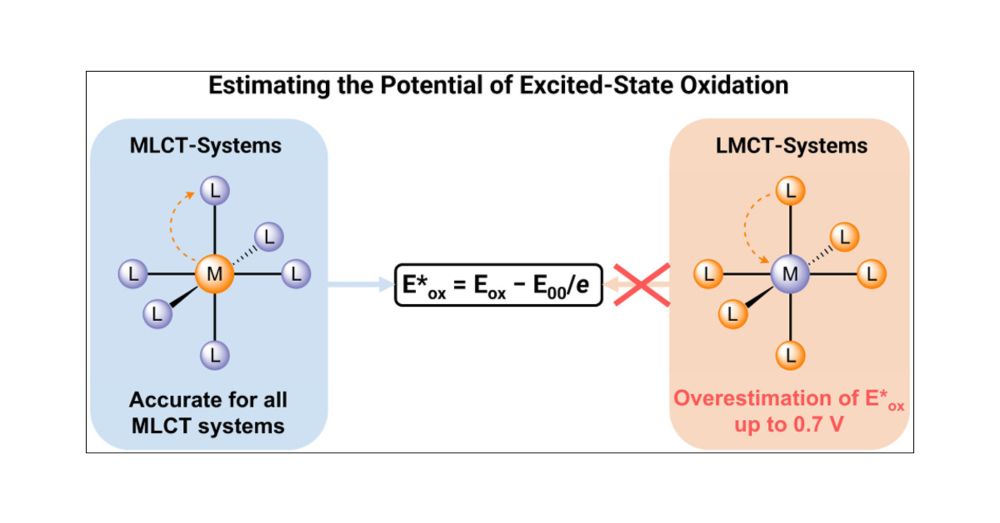
For Fe(III) complexes, excited-state redox potentials don’t follow the usual rules - standard estimation methods fall short
Joël Wellauer with Paul Francis & colleagues at Deakin University in @jacs.acspublications.org
pubs.acs.org/doi/10.1021/...
We compare the σ-donor and π-acceptor properties of fluorinated isocyanide complexes with their non-fluorinated analogues using the EDA-NOCV method.
🔗 pubs.acs.org/doi/10.1021/...
#InorganicChemistry #Organometallics #ComputationalChemistry

We compare the σ-donor and π-acceptor properties of fluorinated isocyanide complexes with their non-fluorinated analogues using the EDA-NOCV method.
🔗 pubs.acs.org/doi/10.1021/...
#InorganicChemistry #Organometallics #ComputationalChemistry
#nanopores #smFRET #biomolecular #dynamics #singleMolecules
We’re looking for talented & ambitious new colleagues enthusiastic about biomolecular dynamics & single-molecule tech.
Please share broadly, thank you!🤝
schmid.chemie.unibas.ch
#nanopores #smFRET #biomolecular #dynamics #singleMolecules
We’re looking for talented & ambitious new colleagues enthusiastic about biomolecular dynamics & single-molecule tech.
Please share broadly, thank you!🤝
schmid.chemie.unibas.ch
pi-donor (instead of pi-acceptor) ligand properties are key
now in @jacs.acspublications.org
pubs.acs.org/doi/10.1021/...
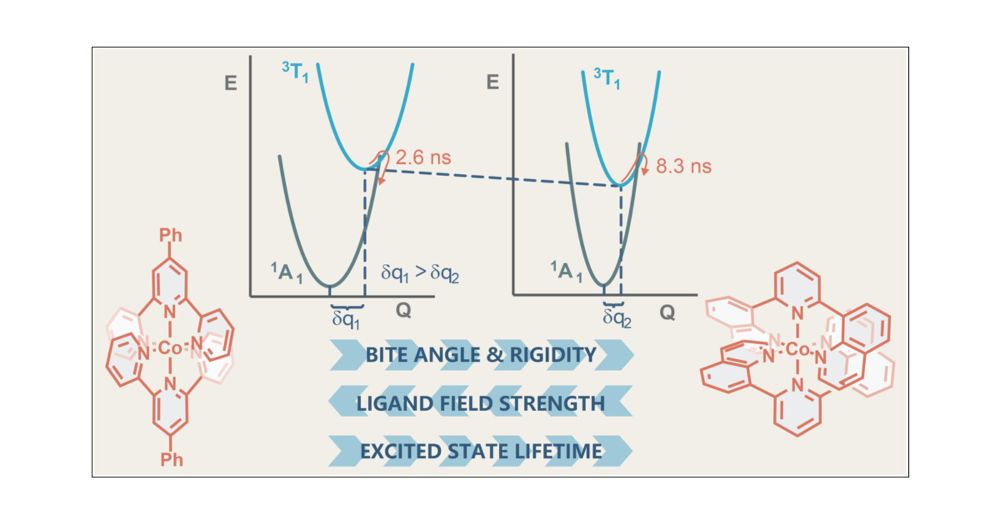
pi-donor (instead of pi-acceptor) ligand properties are key
now in @jacs.acspublications.org
pubs.acs.org/doi/10.1021/...
@giacomo-morselli94.bsky.social & team find signs of doublet–doublet annihilation, or excited-state disproportionation.
In @jacs.acspublications.org
pubs.acs.org/doi/10.1021/...
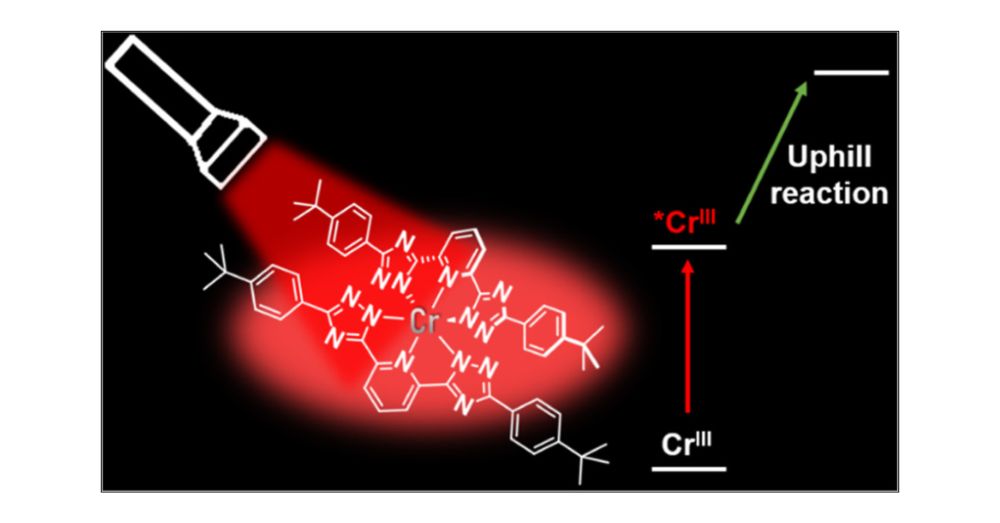
@giacomo-morselli94.bsky.social & team find signs of doublet–doublet annihilation, or excited-state disproportionation.
In @jacs.acspublications.org
pubs.acs.org/doi/10.1021/...
This work, led by the exceptional @bjoernpfund.bsky.social, offers new insights into photochemical reactivity.
Published in JACS @jacs.acspublications.org
bit.ly/3IysmjX
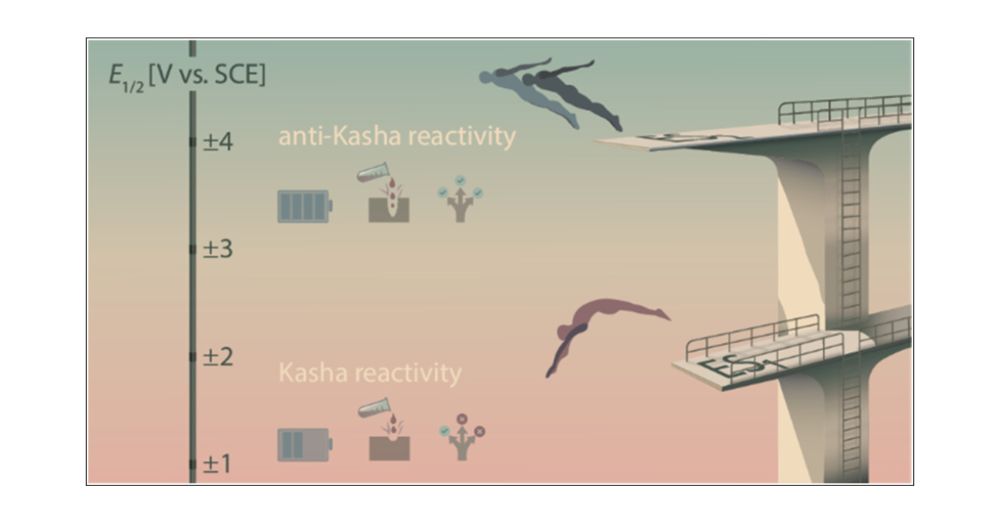
This work, led by the exceptional @bjoernpfund.bsky.social, offers new insights into photochemical reactivity.
Published in JACS @jacs.acspublications.org
bit.ly/3IysmjX
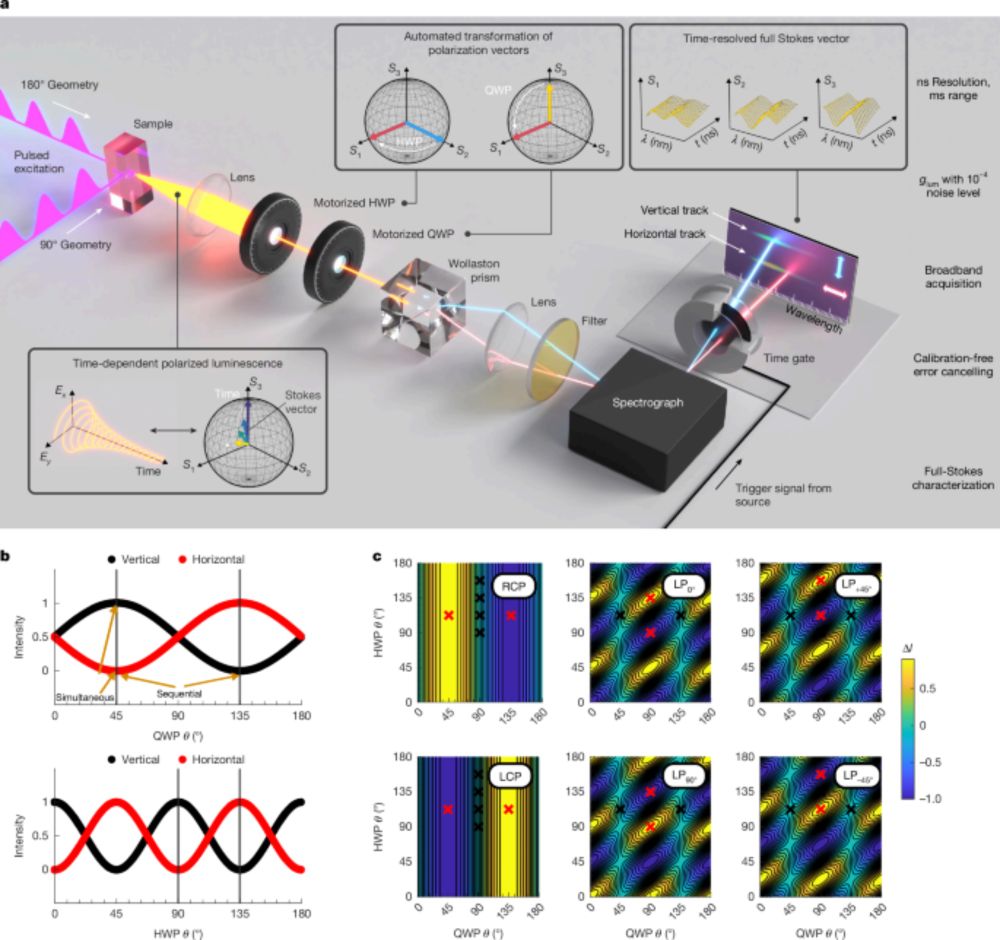
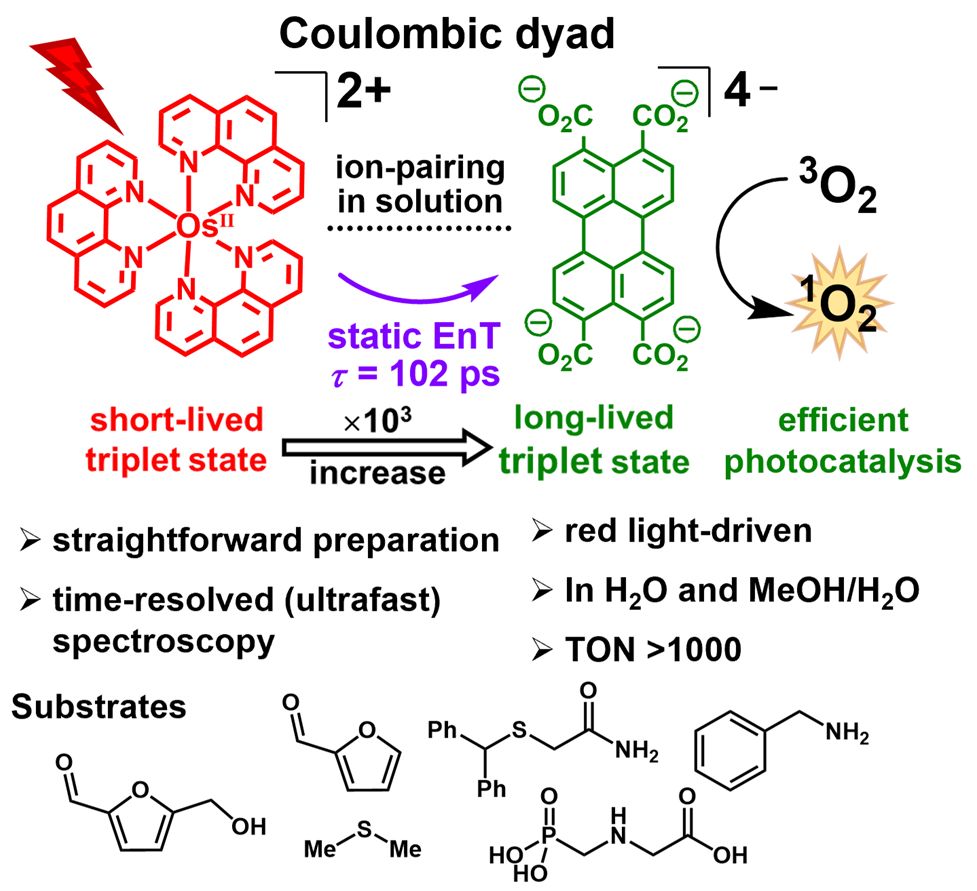
chemrxiv.org/engage/chemr...

chemrxiv.org/engage/chemr...
pubs.acs.org/doi/10.1021/jacs.5c04471 #ChemSky
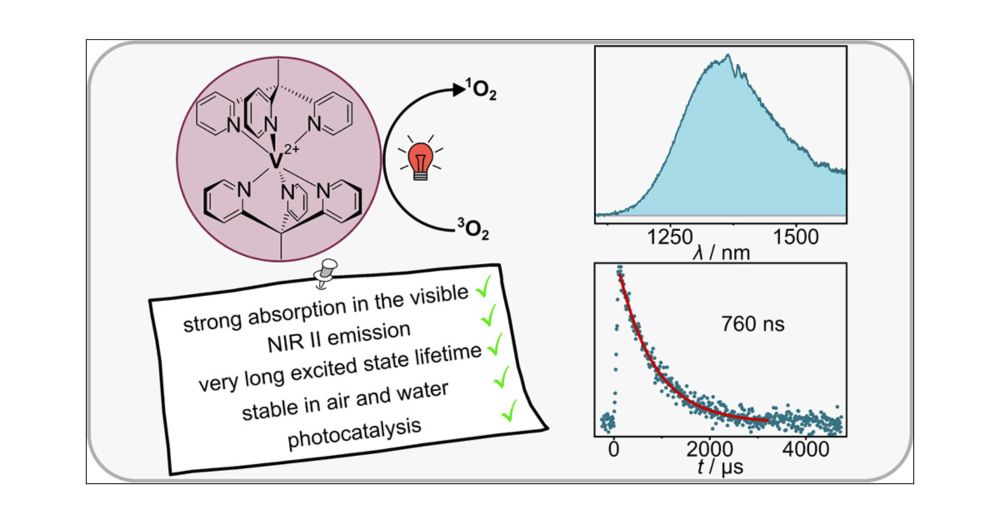
pubs.acs.org/doi/10.1021/jacs.5c04471 #ChemSky

