Photoexcited Manganese Complex of Abnormal N-Heterocyclic Carbene in Molecular Hydrogen Activation via Hydrogen Atom Transfer Pathway #ChemSky
pubs.acs.org/doi/10.1021/...

Photoexcited Manganese Complex of Abnormal N-Heterocyclic Carbene in Molecular Hydrogen Activation via Hydrogen Atom Transfer Pathway #ChemSky
pubs.acs.org/doi/10.1021/...
#ChemSky
pubs.acs.org/doi/10.1021/...

#ChemSky
pubs.acs.org/doi/10.1021/...
Our colleague and friend Armaz Tsutskiridze passed away
We all miss his smile, enthusiasm, politeness and expertise.
Our deepest condolences to his family!

Our colleague and friend Armaz Tsutskiridze passed away
We all miss his smile, enthusiasm, politeness and expertise.
Our deepest condolences to his family!
#ChemSky #photocatalysis #metal-free
pubs.acs.org/doi/10.1021/...

#ChemSky #photocatalysis #metal-free
pubs.acs.org/doi/10.1021/...
@indrajitghosh85.bsky.social
doi.org/10.1002/adsc...

@indrajitghosh85.bsky.social
doi.org/10.1002/adsc...
#photocatalysis #nanoparticles #ChemSky
doi.org/10.1002/anie...
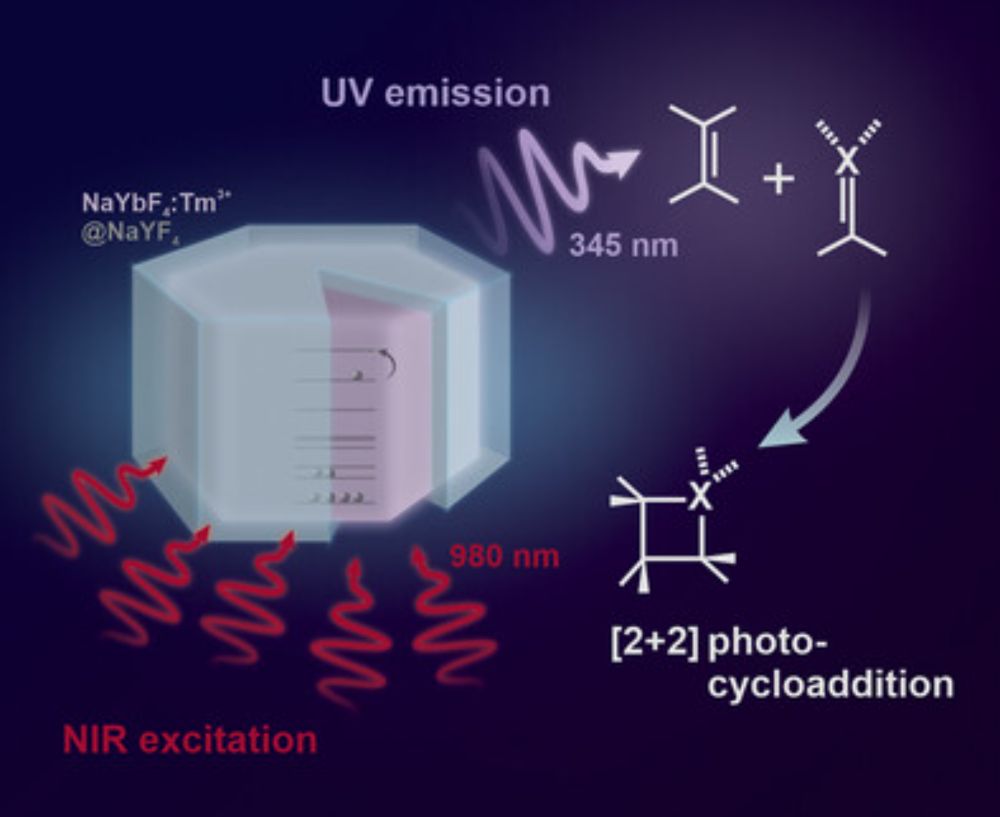
#photocatalysis #nanoparticles #ChemSky
doi.org/10.1002/anie...
This review describes the synthesis, structure–property relationships, and examples of applications for seven important classes of photoswitches
doi.org/10.3762/bjoc...

This review describes the synthesis, structure–property relationships, and examples of applications for seven important classes of photoswitches
doi.org/10.3762/bjoc...
Then use our new strategy to simplify your reactions!
Thanks to all involved!
@angewandtechemie.bsky.social
@indrajitghosh85.bsky.social
#ChemSky #photocatalysis #Nickel
doi.org/10.1002/anie...

Then use our new strategy to simplify your reactions!
Thanks to all involved!
@angewandtechemie.bsky.social
@indrajitghosh85.bsky.social
#ChemSky #photocatalysis #Nickel
doi.org/10.1002/anie...
pubs.acs.org/doi/10.1021/...

pubs.acs.org/doi/10.1021/...
Congrats to Caroline and Michela!
doi.org/10.1002/anie...

Congrats to Caroline and Michela!
doi.org/10.1002/anie...
Please see the prospects for photo-orthogonal synthesis by increasing the conc. in the photostationary state and the lifetime of nonstabilized diazo compounds for stepwise reactions #ChemSky

Please see the prospects for photo-orthogonal synthesis by increasing the conc. in the photostationary state and the lifetime of nonstabilized diazo compounds for stepwise reactions #ChemSky
Congrats to Ranit, Alba and especially Caroline! @pubs.acs.org #ChemSky
Alternative Mechanism of Enzymatic Photocontrol by Azobenzene | ACS Catalysis pubs.acs.org/doi/10.1021/...
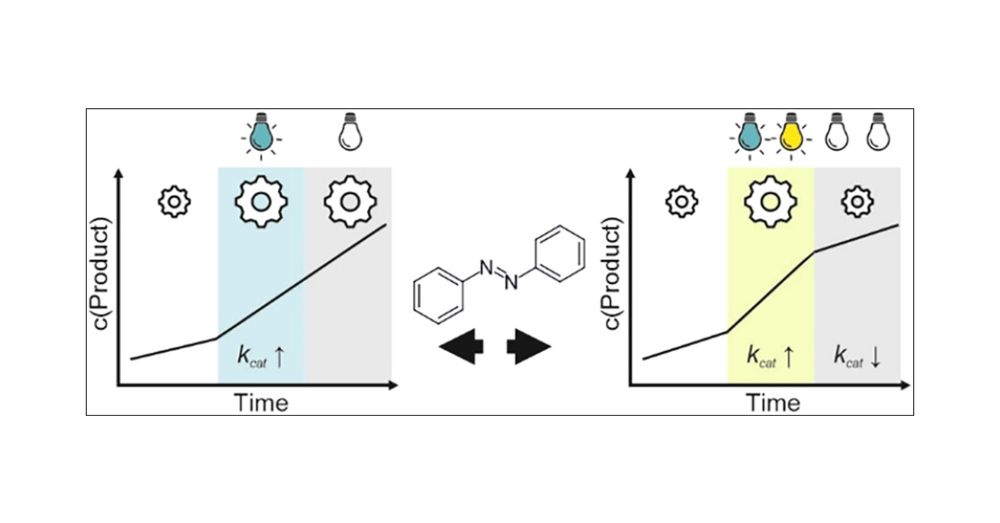
Congrats to Ranit, Alba and especially Caroline! @pubs.acs.org #ChemSky
Alternative Mechanism of Enzymatic Photocontrol by Azobenzene | ACS Catalysis pubs.acs.org/doi/10.1021/...
Magnetic stirrers - used daily in millions of labs - may silently sabotage reproducibility!
pubs.acs.org/doi/10.1021/...
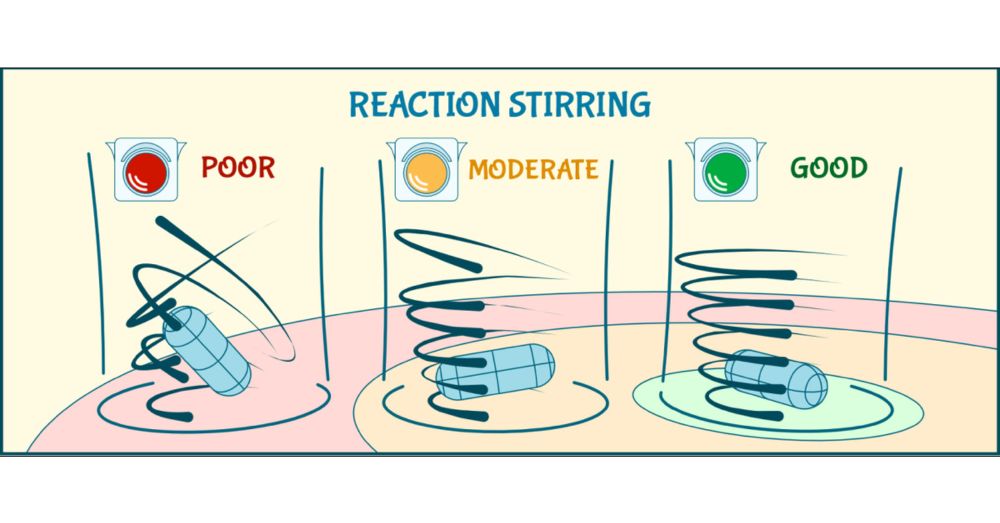
Magnetic stirrers - used daily in millions of labs - may silently sabotage reproducibility!
pubs.acs.org/doi/10.1021/...
Congrats to Nico! #ChemSky #photocatalysis
doi.org/10.1002/ejoc...

Congrats to Nico! #ChemSky #photocatalysis
doi.org/10.1002/ejoc...
doi.org/10.1002/anie...
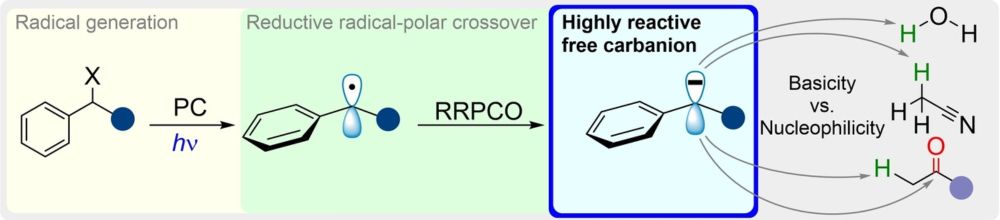
doi.org/10.1002/anie...
Congrats to Daniel and Andrey!
doi.org/10.1002/cptc...
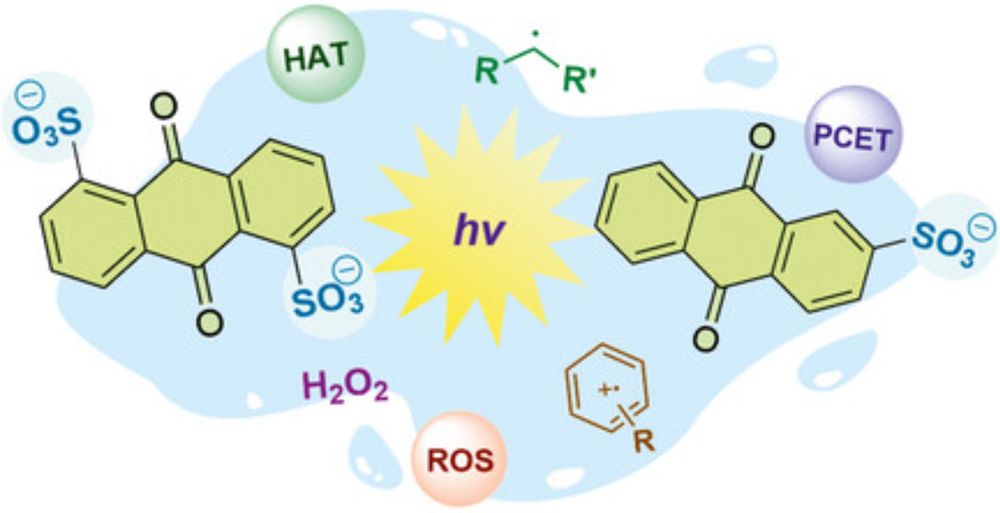
Congrats to Daniel and Andrey!
doi.org/10.1002/cptc...
doi.org/10.1002/chem...
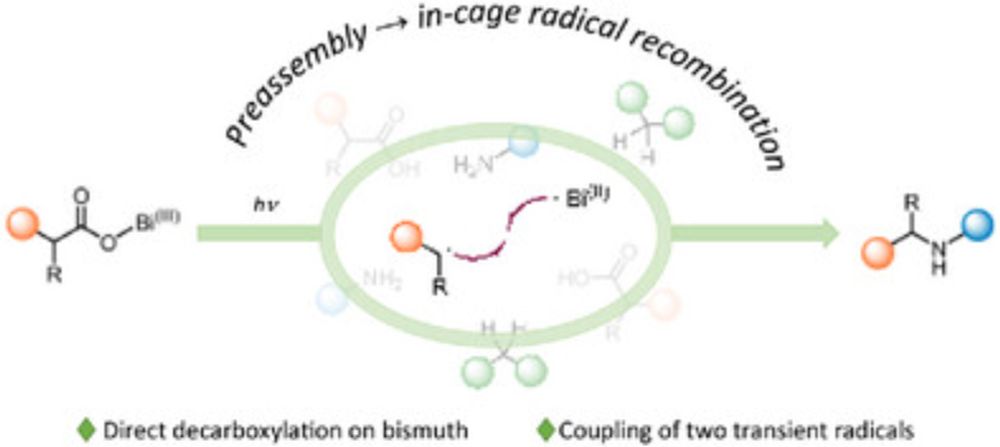
doi.org/10.1002/chem...
doi.org/10.1039/D5CC...

doi.org/10.1039/D5CC...
Congrats to all involved! onlinelibrary.wiley.com/doi/full/10.... #TopCitedArticle @wiley.com

Congrats to all involved! onlinelibrary.wiley.com/doi/full/10.... #TopCitedArticle @wiley.com
Für alle, die Chemie unterrichten:
Die aktuelle Ausgabe von Naturwissenschaften im Unterricht Chemie März 2025, Heft 206, 9-13
www.friedrich-verlag.de/friedrich-pl...
#chemsky #photocatalysis

Für alle, die Chemie unterrichten:
Die aktuelle Ausgabe von Naturwissenschaften im Unterricht Chemie März 2025, Heft 206, 9-13
www.friedrich-verlag.de/friedrich-pl...
#chemsky #photocatalysis
Congratulations to Florence and Indra
@indrajitghosh85.bsky.social
Thanks to the Dixon Group!
#photocatalysis #ChemSky 🧪
www.orgsyn.org/demo.aspx?pr...
Congratulations to Florence and Indra
@indrajitghosh85.bsky.social
Thanks to the Dixon Group!
#photocatalysis #ChemSky 🧪
www.orgsyn.org/demo.aspx?pr...
#ChemSky #photocatalysis
doi.org/10.1002/chem...

#ChemSky #photocatalysis
doi.org/10.1002/chem...
A methodology for achieving complex chemical transformations across a wide range of redox potentials
Congratulations to Lea, Indra and Nuernberger group
#ChemSky #photocatalysis
doi.org/10.1002/chem...

A methodology for achieving complex chemical transformations across a wide range of redox potentials
Congratulations to Lea, Indra and Nuernberger group
#ChemSky #photocatalysis
doi.org/10.1002/chem...

The method’s tolerance to water and acidic environments opens new opportunities for synthetic chemists, facilitating cross-couplings with previously challenging substrates. @pubs.acs.org
#Chemsky #photocatalysis
doi.org/10.1021/acsc...

The method’s tolerance to water and acidic environments opens new opportunities for synthetic chemists, facilitating cross-couplings with previously challenging substrates. @pubs.acs.org
#Chemsky #photocatalysis
doi.org/10.1021/acsc...

