
We show that N₂ can bind to a cationic Mn fragment without donor ligands and explore how this affects N₂ polarization & binding.
Thanks to all coauthors & collaborators!
🔗 doi.org/10.1021/jacs... @jacs.acspublications.org

We show that N₂ can bind to a cationic Mn fragment without donor ligands and explore how this affects N₂ polarization & binding.
Thanks to all coauthors & collaborators!
🔗 doi.org/10.1021/jacs... @jacs.acspublications.org
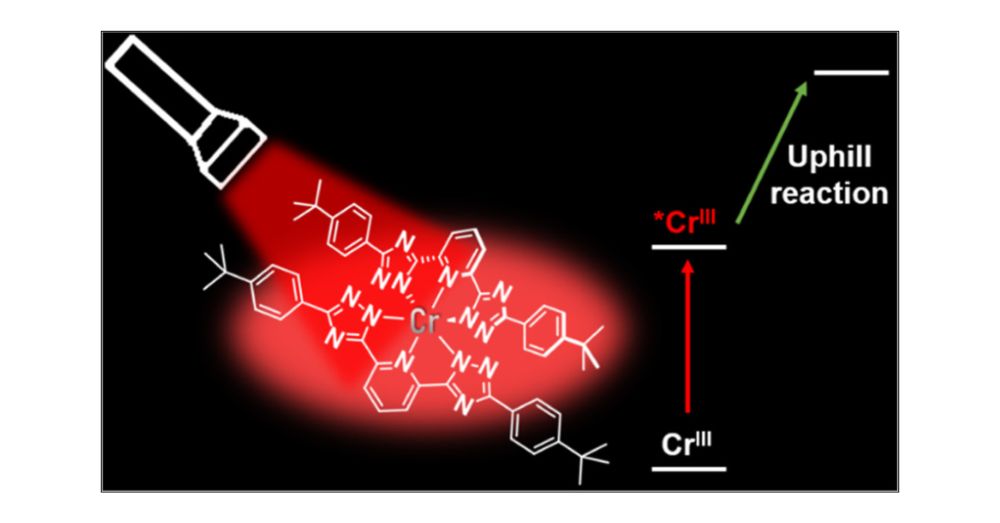
100 ns lifetime, 3 eV energy storage, and 37% quantum yield
A step toward multi-electron photochemistry
Mathis Brändlin and @bjoernpfund.bsky.social in @natchem.nature.com
www.nature.com/articles/s41...

100 ns lifetime, 3 eV energy storage, and 37% quantum yield
A step toward multi-electron photochemistry
Mathis Brändlin and @bjoernpfund.bsky.social in @natchem.nature.com
www.nature.com/articles/s41...

For Fe(III) complexes, excited-state redox potentials don’t follow the usual rules - standard estimation methods fall short
Joël Wellauer with Paul Francis & colleagues at Deakin University in @jacs.acspublications.org
pubs.acs.org/doi/10.1021/...
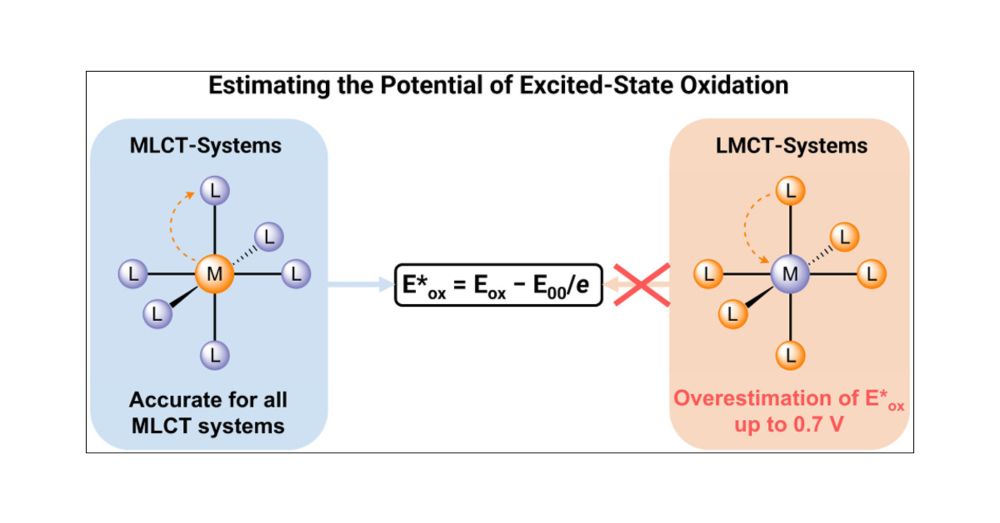
For Fe(III) complexes, excited-state redox potentials don’t follow the usual rules - standard estimation methods fall short
Joël Wellauer with Paul Francis & colleagues at Deakin University in @jacs.acspublications.org
pubs.acs.org/doi/10.1021/...
pi-donor (instead of pi-acceptor) ligand properties are key
now in @jacs.acspublications.org
pubs.acs.org/doi/10.1021/...
pi-donor (instead of pi-acceptor) ligand properties are key
now in @jacs.acspublications.org
pubs.acs.org/doi/10.1021/...
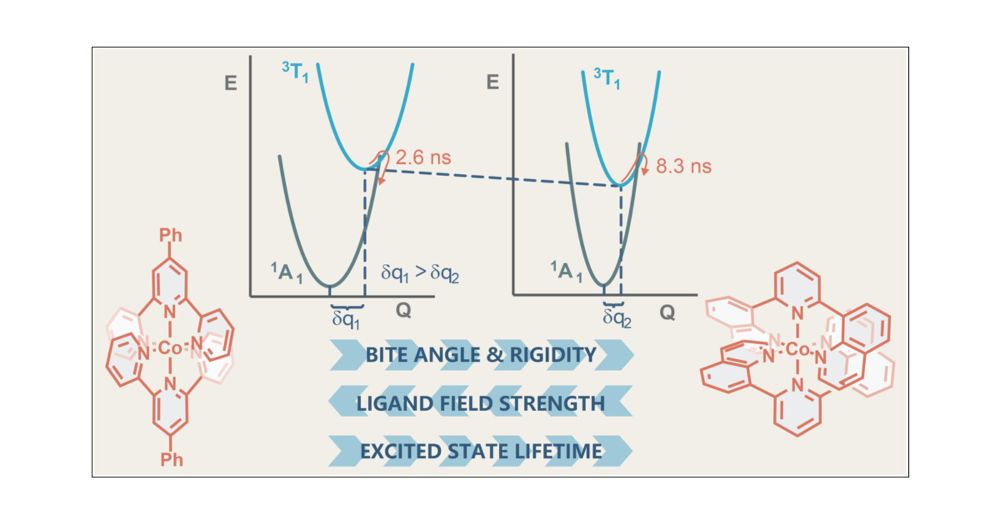
@giacomo-morselli94.bsky.social & team find signs of doublet–doublet annihilation, or excited-state disproportionation.
In @jacs.acspublications.org
pubs.acs.org/doi/10.1021/...
@giacomo-morselli94.bsky.social & team find signs of doublet–doublet annihilation, or excited-state disproportionation.
In @jacs.acspublications.org
pubs.acs.org/doi/10.1021/...
We compare the σ-donor and π-acceptor properties of fluorinated isocyanide complexes with their non-fluorinated analogues using the EDA-NOCV method.
🔗 pubs.acs.org/doi/10.1021/...
#InorganicChemistry #Organometallics #ComputationalChemistry

We compare the σ-donor and π-acceptor properties of fluorinated isocyanide complexes with their non-fluorinated analogues using the EDA-NOCV method.
🔗 pubs.acs.org/doi/10.1021/...
#InorganicChemistry #Organometallics #ComputationalChemistry
CSD entry FUZZEJ contains the first sigma alkane complex to be crystallized from solution.
🔗 ccdc-info.com/3TbaoG4
#FeaturedStructureFriday
CSD entry FUZZEJ contains the first sigma alkane complex to be crystallized from solution.
🔗 ccdc-info.com/3TbaoG4
#FeaturedStructureFriday
doi.org/10.1002/anie...

doi.org/10.1002/anie...
doi.org/10.1002/chem...

doi.org/10.1002/chem...
[Ph3P–PPh3]2+: Superacid, Superoxidant, Super Reagent? pubs.acs.org/doi/10.1021/...
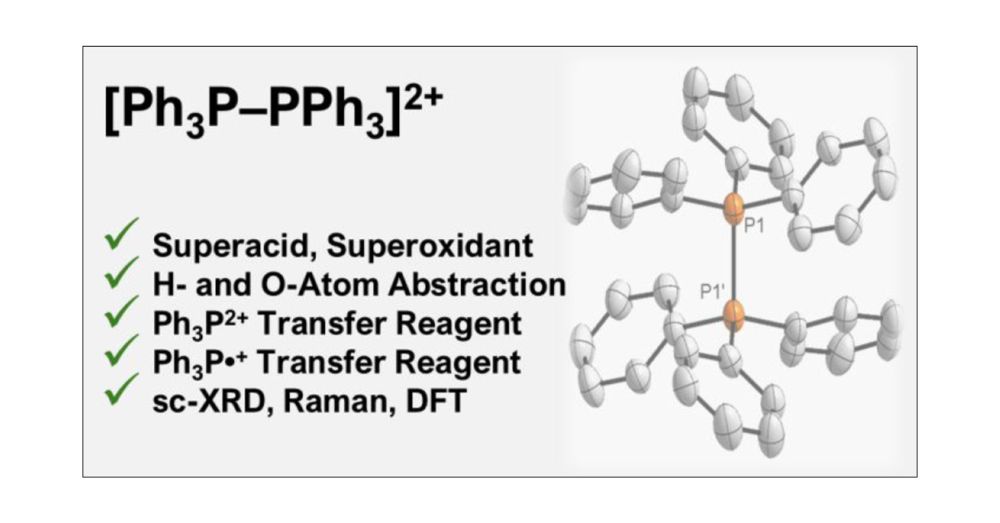
[Ph3P–PPh3]2+: Superacid, Superoxidant, Super Reagent? pubs.acs.org/doi/10.1021/...

With Giacomo Morselli and Christian Reber in JACS @jacs.acspublications.org
pubs.acs.org/doi/10.1021/...
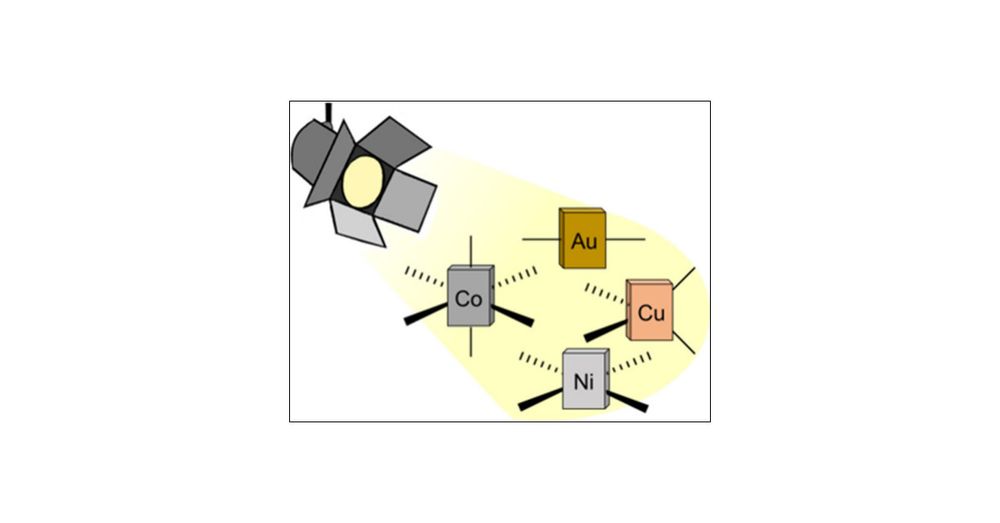
With Giacomo Morselli and Christian Reber in JACS @jacs.acspublications.org
pubs.acs.org/doi/10.1021/...
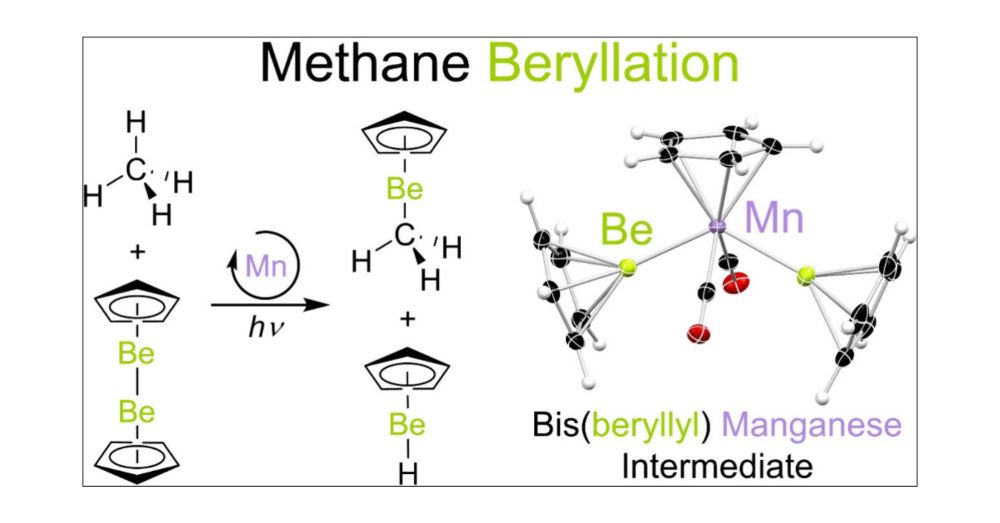
@chemistryviews.bsky.social gave us the opportunity to discuss why we did that and what is cool about it. Be sure to check out the article⬇️
www.chemistryviews.org/first-hetero...

@chemistryviews.bsky.social gave us the opportunity to discuss why we did that and what is cool about it. Be sure to check out the article⬇️

Utilization of an attached organic chromophore while maintaining a luminescent and photoactive LMCT excited state
Joel Wellauer & @bjoernpfund.bsky.social in JACS
pubs.acs.org/doi/10.1021/...
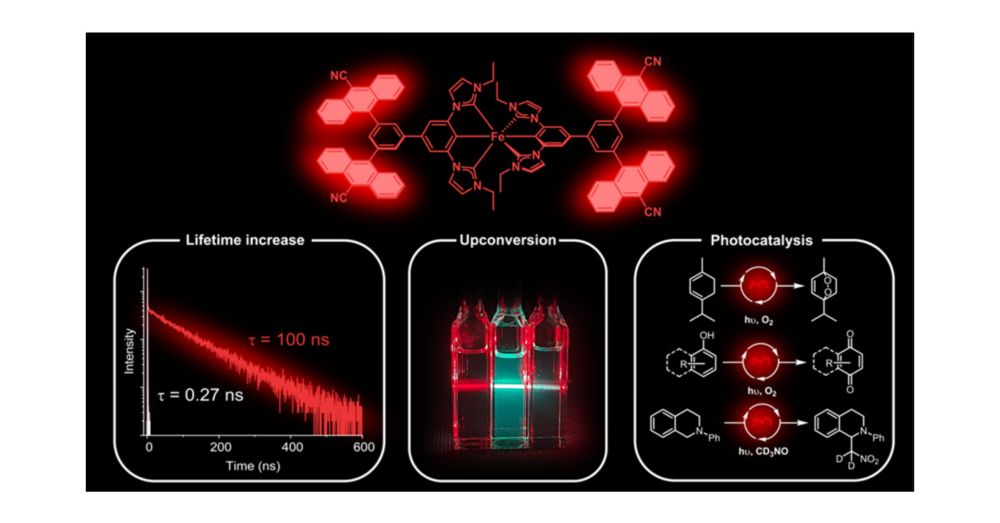
Utilization of an attached organic chromophore while maintaining a luminescent and photoactive LMCT excited state
Joel Wellauer & @bjoernpfund.bsky.social in JACS
pubs.acs.org/doi/10.1021/...

