
Colin Nichols Lab
@colinnicholslab.bsky.social
Posting from Colin Nichols' electrophysiology lab at WashU. Focused on ion channels biophysics & role in physiology and pathology.
Pinned
Discover our work - Colin Nichols Lab
Welcome to the Vitaly Klyachko Lab! We examine synaptic dysfunction in the brain to better understand Alzheimer’s Disease and Fragile Z Syndrome.
nicholslab.wustl.edu
📢 Nichols Lab is hiring! 📢
Join us at WashU & contribute to cutting-edge ion channel research. DM or email
nicholslab.wustl.edu
Join us at WashU & contribute to cutting-edge ion channel research. DM or email
nicholslab.wustl.edu
Reposted by Colin Nichols Lab
Muscle fatigue arising intrinsically from SUR2- but not Kir6.1-dependent gain-of-function in Cantu syndrome mice. A new study from Rosa Scala, Colin Nichols et al. @colinnicholslab.bsky.social rupress.org/jgp/article/...
#IonChannels #MolecularPhysiology #Pathophysiology #SkeletalMuscle
#IonChannels #MolecularPhysiology #Pathophysiology #SkeletalMuscle
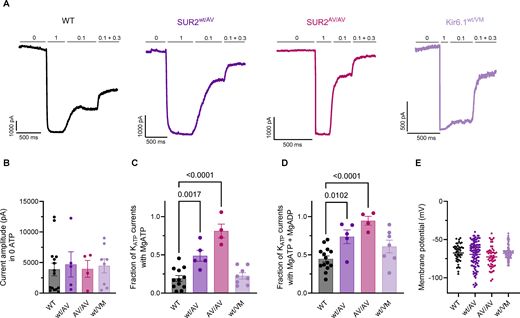
Muscle fatigue arising intrinsically from SUR2- but not Kir6.1-dependent gain-of-function in Cantu syndrome mice
We assessed skeletal muscle properties in GOF knock-in mouse models of Cantu Syndrome. In isolated myofibers there was enhanced Mg-nucleotide activation in
rupress.org
October 14, 2025 at 2:13 PM
Muscle fatigue arising intrinsically from SUR2- but not Kir6.1-dependent gain-of-function in Cantu syndrome mice. A new study from Rosa Scala, Colin Nichols et al. @colinnicholslab.bsky.social rupress.org/jgp/article/...
#IonChannels #MolecularPhysiology #Pathophysiology #SkeletalMuscle
#IonChannels #MolecularPhysiology #Pathophysiology #SkeletalMuscle
Reposted by Colin Nichols Lab
PIP2-driven cytoplasmic domain motions are coupled to Kir2 channel gating, say Eva-Maria Zangerl-Plessl, Anna Stary-Weinzinger, Colin G. Nichols, and Sun-Joo Lee rupress.org/jgp/article/...
@colinnicholslab.bsky.social
#IonChannels #Phospholipids
@colinnicholslab.bsky.social
#IonChannels #Phospholipids

October 10, 2025 at 1:26 PM
PIP2-driven cytoplasmic domain motions are coupled to Kir2 channel gating, say Eva-Maria Zangerl-Plessl, Anna Stary-Weinzinger, Colin G. Nichols, and Sun-Joo Lee rupress.org/jgp/article/...
@colinnicholslab.bsky.social
#IonChannels #Phospholipids
@colinnicholslab.bsky.social
#IonChannels #Phospholipids
Check out our new publication "Treatment of overactive KATP channels with glibenclamide in a zebrafish model and a clinical trial in humans with Cantú syndrome" 🧪 @nature.com
www.nature.com/articles/s41...
www.nature.com/articles/s41...

Treatment of overactive KATP channels with glibenclamide in a zebrafish model and a clinical trial in humans with Cantú syndrome - Scientific Reports
Scientific Reports - Treatment of overactive KATP channels with glibenclamide in a zebrafish model and a clinical trial in humans with Cantú syndrome
www.nature.com
October 7, 2025 at 9:05 PM
Check out our new publication "Treatment of overactive KATP channels with glibenclamide in a zebrafish model and a clinical trial in humans with Cantú syndrome" 🧪 @nature.com
www.nature.com/articles/s41...
www.nature.com/articles/s41...
Check out our new publication "Molecular basis of TRPV3 channel blockade by intracellular polyamines" 🧪
www.nature.com/articles/s42...
www.nature.com/articles/s42...

Molecular basis of TRPV3 channel blockade by intracellular polyamines - Communications Biology
Identification of TRPV3 channel residues interacting with intracellular spermine and high resolution structure of a non-conducting TRPV3 in the presence of NASPM suggest a unifying molecular model to explain spermine block of TRPV1-4 channels.
www.nature.com
October 7, 2025 at 9:02 PM
Check out our new publication "Molecular basis of TRPV3 channel blockade by intracellular polyamines" 🧪
www.nature.com/articles/s42...
www.nature.com/articles/s42...
Reposted by Colin Nichols Lab
In @jgp.org, Scala et al @colinnicholslab.bsky.social assess #SkeletalMuscle properties in gain-of-function knock-in mouse models of Cantu Syndrome. Isolated SUR2 GOF, but not Kir6.1 GOF muscles show enhanced fatiguing that was reversed by the KATP inhibitor glibenclamide

Muscle fatigue arising intrinsically from SUR2- but not Kir6.1-dependent gain-of-function in Cantu syndrome mice
We assessed skeletal muscle properties in GOF knock-in mouse models of Cantu Syndrome. In isolated myofibers there was enhanced Mg-nucleotide activation in
rupress.org
October 7, 2025 at 7:12 PM
In @jgp.org, Scala et al @colinnicholslab.bsky.social assess #SkeletalMuscle properties in gain-of-function knock-in mouse models of Cantu Syndrome. Isolated SUR2 GOF, but not Kir6.1 GOF muscles show enhanced fatiguing that was reversed by the KATP inhibitor glibenclamide
Check out our new publication "Muscle fatigue arising intrinsically from SUR2- but not Kir6.1-dependent gain-of-function in Cantu syndrome mice" 🧪 @rosca26.bsky.social
A 🧵 1/n
A 🧵 1/n
Scala et al. @colinnicholslab.bsky.social assess #SkeletalMuscle properties in gain-of-function knock-in mouse models of Cantu Syndrome. Isolated SUR2 GOF, but not Kir6.1 GOF muscles showed enhanced fatiguing that was reversed by the KATP channel inhibitor glibenclamide.

Muscle fatigue arising intrinsically from SUR2- but not Kir6.1-dependent gain-of-function in Cantu syndrome mice
We assessed skeletal muscle properties in GOF knock-in mouse models of Cantu Syndrome. In isolated myofibers there was enhanced Mg-nucleotide activation in
rupress.org
October 7, 2025 at 8:57 PM
Check out our new publication "Muscle fatigue arising intrinsically from SUR2- but not Kir6.1-dependent gain-of-function in Cantu syndrome mice" 🧪 @rosca26.bsky.social
A 🧵 1/n
A 🧵 1/n
Reposted by Colin Nichols Lab
New in @jgp.org: Zangerl-Plessl, Lee, et al. utilized MD simulations to reveal that PIP2 potentiated clockwise twisting motions in individual Kir2 #IonChannel cytoplasmic subunits, as well as concerted dynamics among the four subunits. rupress.org/jgp/article/...
@colinnicholslab.bsky.social
@colinnicholslab.bsky.social

October 3, 2025 at 5:18 PM
New in @jgp.org: Zangerl-Plessl, Lee, et al. utilized MD simulations to reveal that PIP2 potentiated clockwise twisting motions in individual Kir2 #IonChannel cytoplasmic subunits, as well as concerted dynamics among the four subunits. rupress.org/jgp/article/...
@colinnicholslab.bsky.social
@colinnicholslab.bsky.social
Check out our new publication "PIP2-driven cytoplasmic domain motions are coupled to Kir2 channel gating" 🧪
A 🧵 1/n
A 🧵 1/n
Eva-Maria Zangerl-Plessl, Sun-Joo Lee, et al. utilized MD simulations to reveal that PIP2 potentiated clockwise twisting motions in individual Kir2 #IonChannel cytoplasmic subunits, as well as concerted dynamics among the four subunits. rupress.org/jgp/article/...
@colinnicholslab.bsky.social
@colinnicholslab.bsky.social

October 7, 2025 at 8:40 PM
Check out our new publication "PIP2-driven cytoplasmic domain motions are coupled to Kir2 channel gating" 🧪
A 🧵 1/n
A 🧵 1/n
We love it Stephen, thank you! 🧪
Dr. Colin G. Nichols shared how the KATP channel drives both neonatal diabetes & congenital hyperinsulinism. 🧬 Key insights: SUR1 mutations, shifts in calcium sensitivity, & why diazoxide works (when sulfonylureas may not).
@washumedicine.bsky.social @colinnicholslab.bsky.social #EndoSky
@washumedicine.bsky.social @colinnicholslab.bsky.social #EndoSky

October 7, 2025 at 8:32 PM
We love it Stephen, thank you! 🧪
Check out our new publication "Paradoxical Maturity-Onset Diabetes of the Young Arising From Loss-of-Function Mutations in ATP-Sensitive Potassium Channels" 🧪 diabetesjournals.org/diabetes/art... @rosca26.bsky.social
A 🧵 1/n
A 🧵 1/n

Paradoxical Maturity-Onset Diabetes of the Young Arising From Loss-of-Function Mutations in ATP-Sensitive Potassium Channels
Pancreatic β-cell ATP-sensitive K+ (KATP) channel closure underlies electrical excitability and insulin release, but loss or inhibition of KATP channels ca
diabetesjournals.org
May 22, 2025 at 6:56 PM
Check out our new publication "Paradoxical Maturity-Onset Diabetes of the Young Arising From Loss-of-Function Mutations in ATP-Sensitive Potassium Channels" 🧪 diabetesjournals.org/diabetes/art... @rosca26.bsky.social
A 🧵 1/n
A 🧵 1/n
Next Monday! 🧪

May 17, 2025 at 1:44 PM
Next Monday! 🧪
Join us today for a new exciting CIMED seminar! 🧪 @osamaharraz.bsky.social

May 5, 2025 at 3:13 PM
Join us today for a new exciting CIMED seminar! 🧪 @osamaharraz.bsky.social
Join us today for a new exciting CIMED seminar! 🧪

April 7, 2025 at 3:24 PM
Join us today for a new exciting CIMED seminar! 🧪
🧪
Dynein light chains 1 and 2 are auxiliary proteins of pH-sensitive Kir4.1 channels pubmed.ncbi.nlm.nih.gov/40074079/ #cryoem
March 17, 2025 at 1:37 PM
🧪
Check out our new publication "Dynein light chains 1 and 2 are auxiliary proteins of pH-sensitive Kir4.1 channels"
www.sciencedirect.com/science/arti...
A 🧵 1/n
www.sciencedirect.com/science/arti...
A 🧵 1/n

Dynein light chains 1 and 2 are auxiliary proteins of pH-sensitive Kir4.1 channels
Inward rectifier Kir4.1 potassium channels are abundantly expressed in cells that are important for electrolyte homeostasis. Dysregulation of Kir4.1 u…
www.sciencedirect.com
March 15, 2025 at 2:24 PM
Check out our new publication "Dynein light chains 1 and 2 are auxiliary proteins of pH-sensitive Kir4.1 channels"
www.sciencedirect.com/science/arti...
A 🧵 1/n
www.sciencedirect.com/science/arti...
A 🧵 1/n
Join us today for a new exciting CIMED seminar! 🧪

March 3, 2025 at 4:15 PM
Join us today for a new exciting CIMED seminar! 🧪
Join us today for a new exciting CIMED seminar!

February 13, 2025 at 3:55 PM
Join us today for a new exciting CIMED seminar!
📢 Nichols Lab is hiring! 📢
Join us at WashU & contribute to cutting-edge ion channel research. DM or email
nicholslab.wustl.edu
Join us at WashU & contribute to cutting-edge ion channel research. DM or email
nicholslab.wustl.edu
Discover our work - Colin Nichols Lab
Welcome to the Vitaly Klyachko Lab! We examine synaptic dysfunction in the brain to better understand Alzheimer’s Disease and Fragile Z Syndrome.
nicholslab.wustl.edu
January 28, 2025 at 3:35 PM
📢 Nichols Lab is hiring! 📢
Join us at WashU & contribute to cutting-edge ion channel research. DM or email
nicholslab.wustl.edu
Join us at WashU & contribute to cutting-edge ion channel research. DM or email
nicholslab.wustl.edu
Check out our new publication "Control of neurovascular coupling by ATP-sensitive potassium channels"
A 🧵 1
journals.sagepub.com/doi/10.1177/...
A 🧵 1
journals.sagepub.com/doi/10.1177/...
journals.sagepub.com
January 23, 2025 at 3:49 PM
Check out our new publication "Control of neurovascular coupling by ATP-sensitive potassium channels"
A 🧵 1
journals.sagepub.com/doi/10.1177/...
A 🧵 1
journals.sagepub.com/doi/10.1177/...

