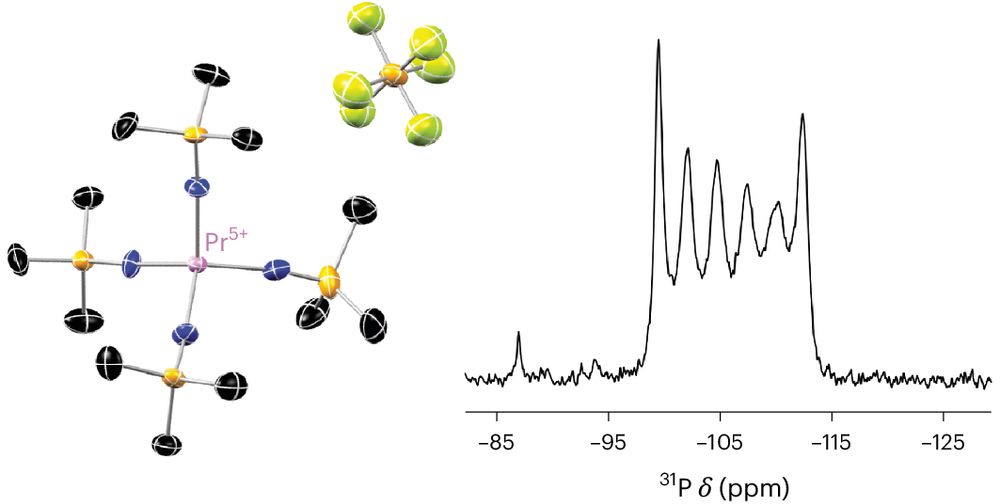
The 5+ state was achieved through oxidation of the tetravalent imidophosphorane complex.
🔗CSD Entry TUNXOT: ccdc-info.com/4je9YdB
#FeaturedStructureFriday
@natchem.nature.com
The 5+ state was achieved through oxidation of the tetravalent imidophosphorane complex.
🔗CSD Entry TUNXOT: ccdc-info.com/4je9YdB
#FeaturedStructureFriday
@natchem.nature.com
https://go.nature.com/4jRn6FB

https://go.nature.com/4jRn6FB
unr.zoom.us/meeting/regi... @acs.org

unr.zoom.us/meeting/regi... @acs.org
www.nature.com/articles/s41...
www.nature.com/articles/s41...
https://bit.ly/4c7UqFt
#chemsky

https://bit.ly/4c7UqFt
#chemsky
Learn more: buff.ly/3XnrNxI

Learn more: buff.ly/3XnrNxI
@univbordeaux.bsky.social aux.bsky.social. Contact us ASAP, @cleracr.bsky.social

@univbordeaux.bsky.social aux.bsky.social. Contact us ASAP, @cleracr.bsky.social
In our editorial this week, we look back to 1925
🧪
@nature.com @natureportfolio.nature.com
www.nature.com/articles/d41...

In our editorial this week, we look back to 1925
🧪
@nature.com @natureportfolio.nature.com
www.nature.com/articles/d41...

I've been very intrigued by the prospect of isolating this unusual tetrahomoleptic U6+ di BArF salt for some time, and am happy to share it in IC! s/o to my co authors!
chemsky⚗️
pubs.acs.org/doi/10.1021/...
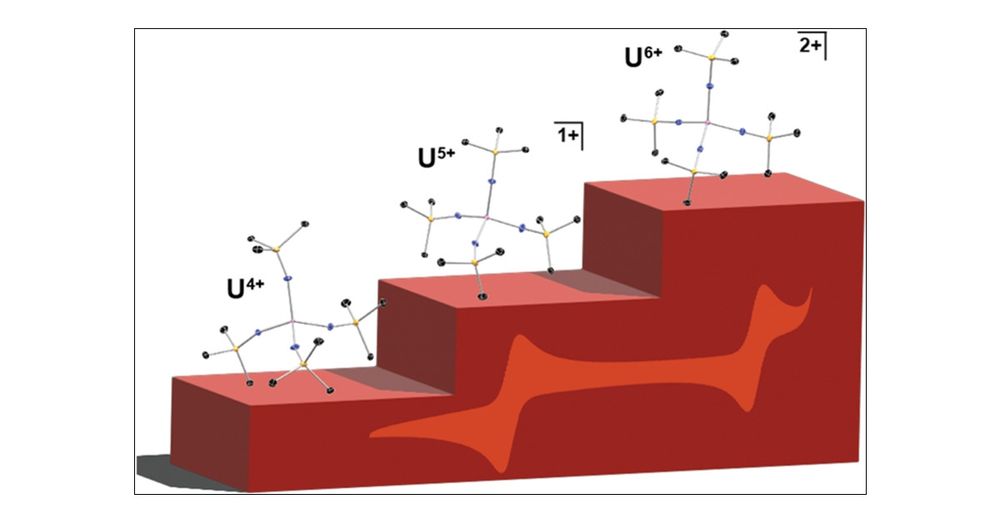
I've been very intrigued by the prospect of isolating this unusual tetrahomoleptic U6+ di BArF salt for some time, and am happy to share it in IC! s/o to my co authors!
chemsky⚗️
pubs.acs.org/doi/10.1021/...
❓❓❓ Who are we❓❓❓




pubs.acs.org/doi/10.1021/...

pubs.acs.org/doi/10.1021/...
-Lectures from National Lab Researchers
-networking, grad school Q&As, lab tours, and more
- Apply by Feb.15th: trucorechemistry.com/apply

cen.acs.org/people/obitu...
cen.acs.org/people/obitu...
I don't want touchscreens, I don't want wifi integration, I don't want centrifuges demanding to know what time zone they're in.
I just want reliable instruments - with physical buttons - that do the one task they were made for.
I don't want touchscreens, I don't want wifi integration, I don't want centrifuges demanding to know what time zone they're in.
I just want reliable instruments - with physical buttons - that do the one task they were made for.
I don't want touchscreens, I don't want wifi integration, I don't want centrifuges demanding to know what time zone they're in.
I just want reliable instruments - with physical buttons - that do the one task they were made for.