Professor, Georgia Institute of Technology
https://www.lapierregroup.com/
https://www.trucorechemistry.com/
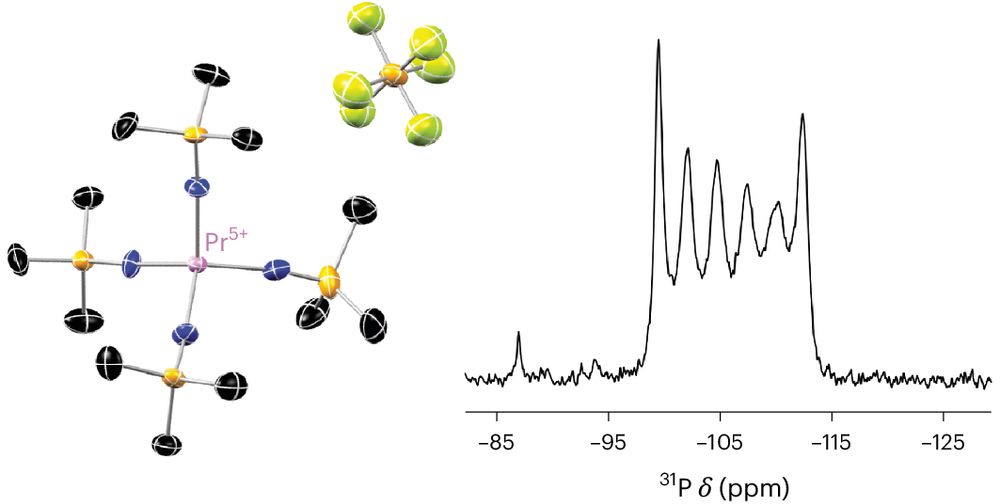
Find our highlight "A high five for praseodymium" following this link
www.nature.com/articles/s41...
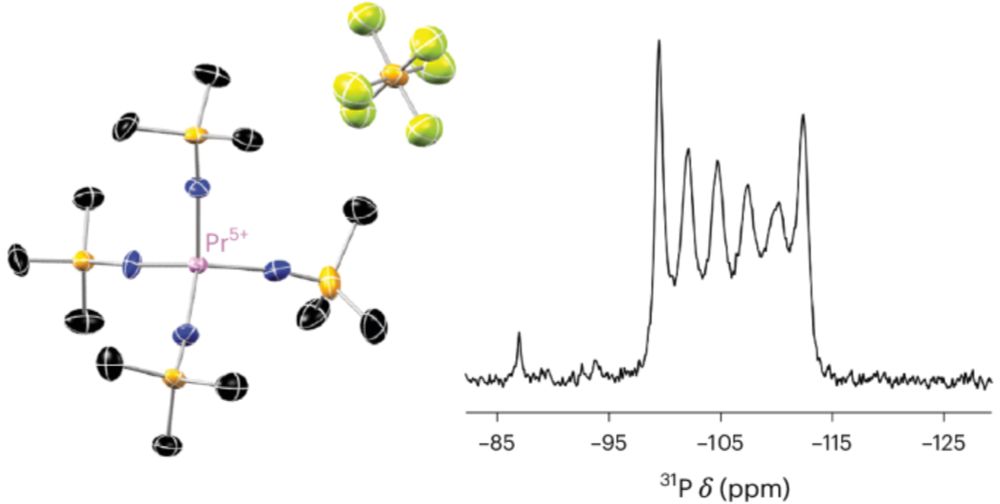
Find our highlight "A high five for praseodymium" following this link
www.nature.com/articles/s41...
www.nature.com/articles/d41...

www.nature.com/articles/d41...
2021 - 2074
2022 - 2193
2023 - 2555
2024 - 2036
2025 - 1000
Again, these funding cuts will have drastic longterm effects on the scientific future of the US.......
@jeremymberg.bsky.social @altnih4science.bsky.social
2021 - 2074
2022 - 2193
2023 - 2555
2024 - 2036
2025 - 1000
Again, these funding cuts will have drastic longterm effects on the scientific future of the US.......
@jeremymberg.bsky.social @altnih4science.bsky.social


I've been very intrigued by the prospect of isolating this unusual tetrahomoleptic U6+ di BArF salt for some time, and am happy to share it in IC! s/o to my co authors!
chemsky⚗️
pubs.acs.org/doi/10.1021/...
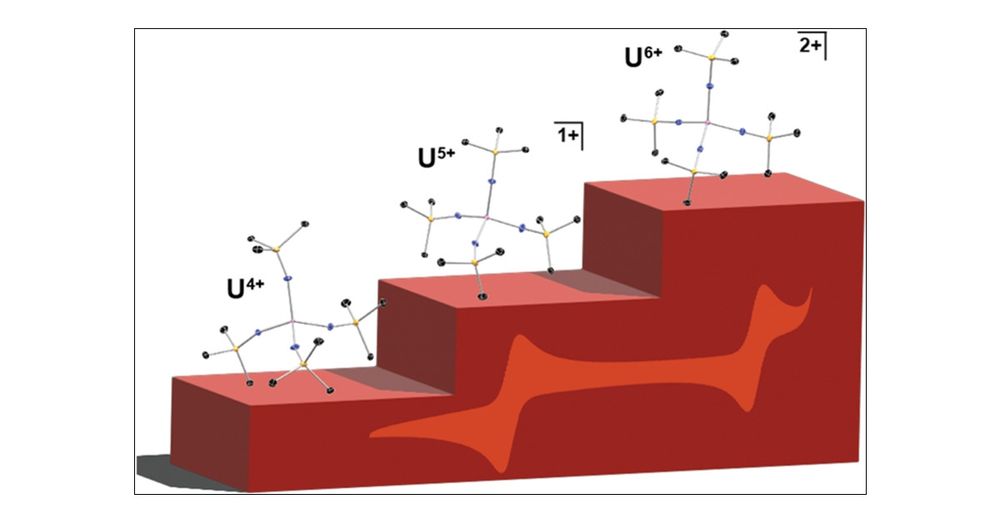
I've been very intrigued by the prospect of isolating this unusual tetrahomoleptic U6+ di BArF salt for some time, and am happy to share it in IC! s/o to my co authors!
chemsky⚗️
pubs.acs.org/doi/10.1021/...
-Lectures from National Lab Researchers
-networking, grad school Q&As, lab tours, and more
- Apply by Feb.15th: trucorechemistry.com/apply

-Lectures from National Lab Researchers
-networking, grad school Q&As, lab tours, and more
- Apply by Feb.15th: trucorechemistry.com/apply