
@chemrxiv.bsky.social go.shr.lc/4j8O5MM

@chemrxiv.bsky.social go.shr.lc/4j8O5MM
pubs.acs.org/doi/10.1021/...
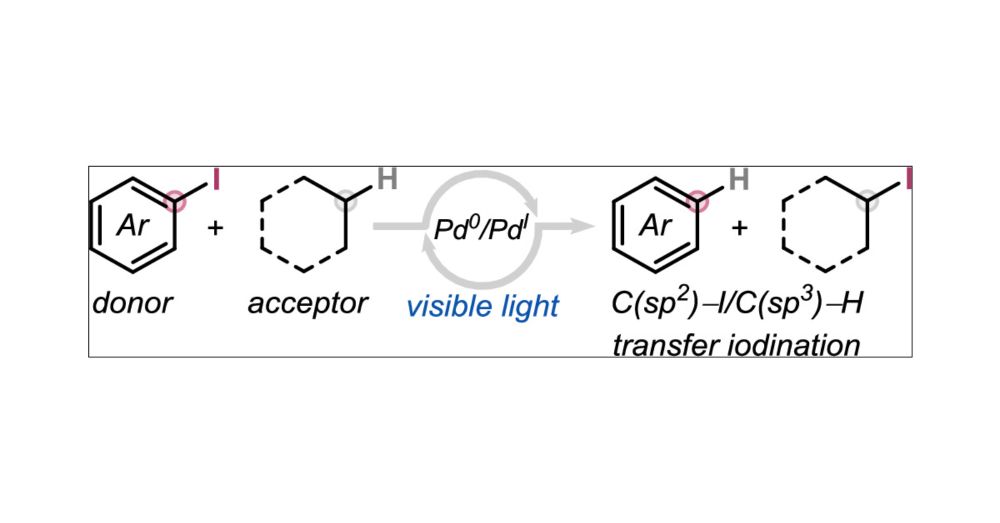
pubs.acs.org/doi/10.1021/...
Ann-Sophie Paschke, Nima Nasiri, Bence Botlik
and Francesco Felician for this masterpiece! Oxidative amination by nitrogen atom insertion into carbon-carbon double bonds in Science, @ethzurich.bsky.social.
www.science.org/doi/10.1126/...
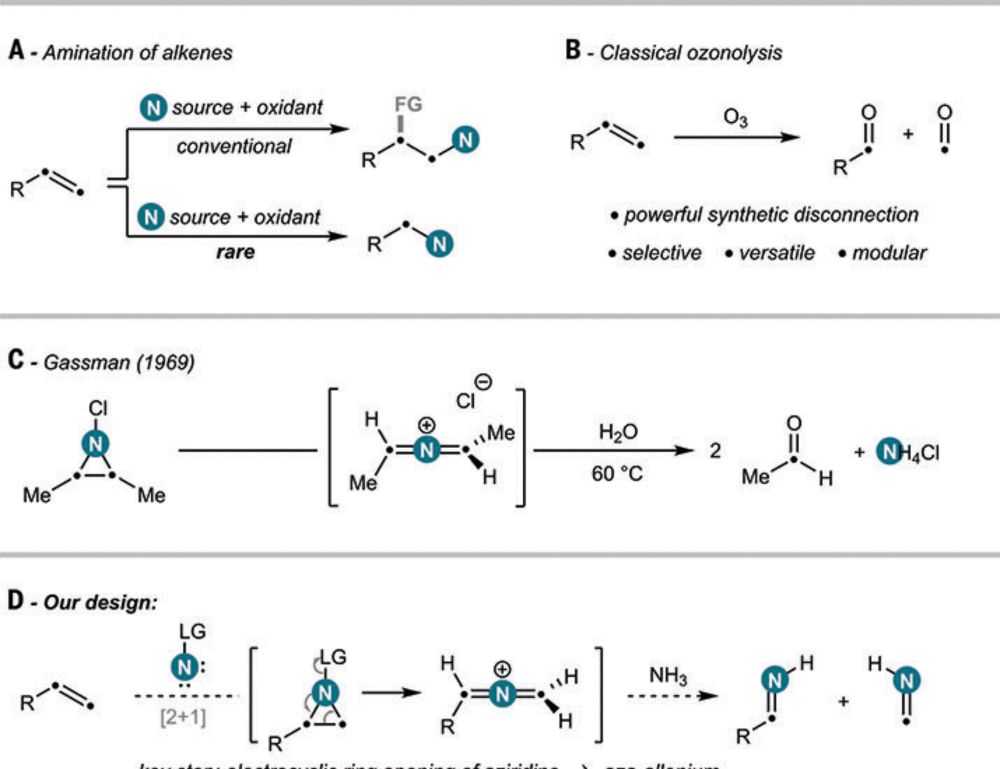
Ann-Sophie Paschke, Nima Nasiri, Bence Botlik
and Francesco Felician for this masterpiece! Oxidative amination by nitrogen atom insertion into carbon-carbon double bonds in Science, @ethzurich.bsky.social.
www.science.org/doi/10.1126/...
www.science.org/doi/10.1126/...
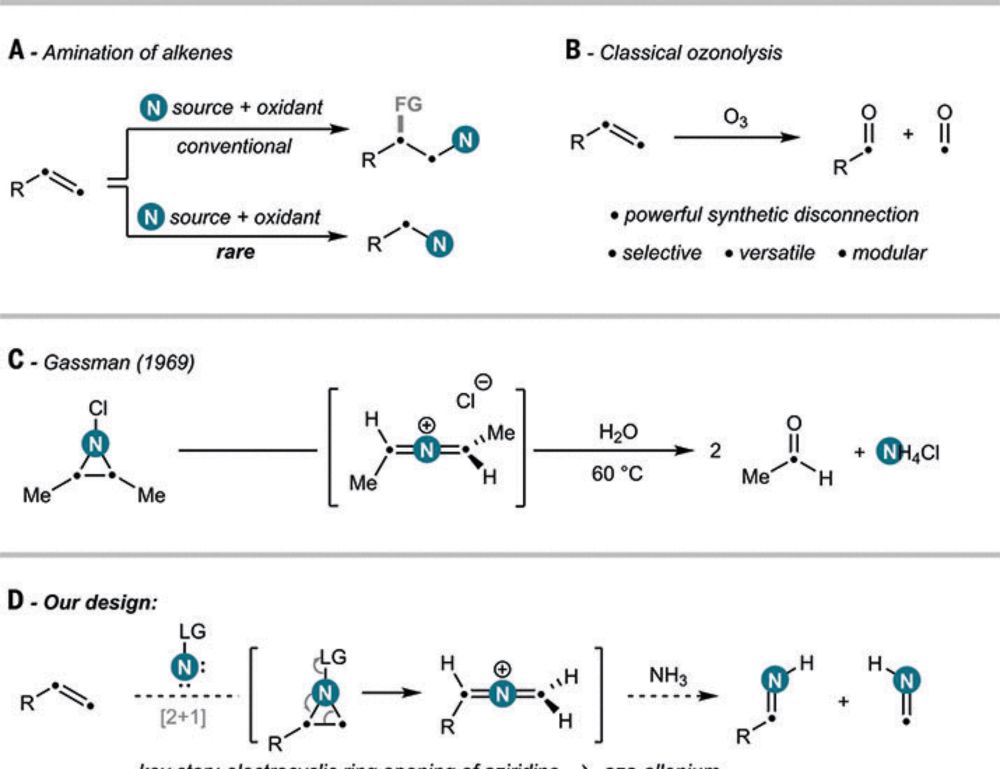
www.science.org/doi/10.1126/...

www.nature.com/articles/s41...
I watched Sven lead this work experimentally and computationally and was very impressed - very rare for someone to be so adept at both. Great to see his hard work pay off.
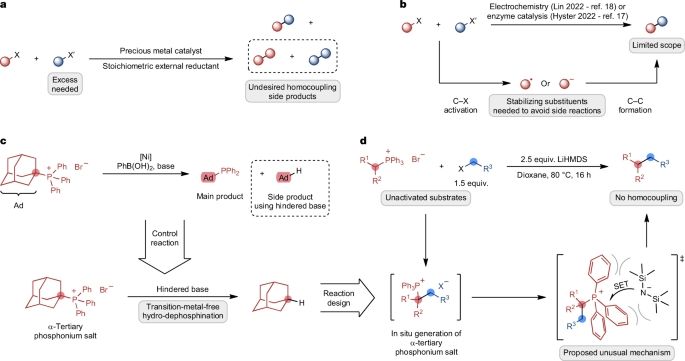
www.nature.com/articles/s41...
I watched Sven lead this work experimentally and computationally and was very impressed - very rare for someone to be so adept at both. Great to see his hard work pay off.

