Ribbe Hu Labs
@ribbehulab.bsky.social
Metalloprotein assembly and catalysis; bioinorganic chemistry; structural biology; spectroscopy; microbiology
Pinned

Assistant Professor in Structural Biology and Biochemistry, Department of Molecular Biology & Biochemistry
University of California, Irvine is hiring. Apply now!
recruit.ap.uci.edu
Our home department of Molecular Biology & Biochemistry at UC Irvine is looking to hire a new tenure-track assistant professor in the broad area of structural biology. Come be our colleague! recruit.ap.uci.edu/JPF09887
Please apply and/or share this post.
Please apply and/or share this post.
Reposted by Ribbe Hu Labs
Our latest work on the nitrogenase-like methylthio-alkane reductase, which specifically reduces reduces carbon-sulfide bonds is now out @natcatal.nature.com: doi.org/10.1038/s419.... We find for the first time large #nitrogenase metalloclusters (P- and L-cluster) outside nitrogenases.
October 23, 2025 at 10:09 AM
Our latest work on the nitrogenase-like methylthio-alkane reductase, which specifically reduces reduces carbon-sulfide bonds is now out @natcatal.nature.com: doi.org/10.1038/s419.... We find for the first time large #nitrogenase metalloclusters (P- and L-cluster) outside nitrogenases.
Our home department of Molecular Biology & Biochemistry at UC Irvine is looking to hire a new tenure-track assistant professor in the broad area of structural biology. Come be our colleague! recruit.ap.uci.edu/JPF09887
Please apply and/or share this post.
Please apply and/or share this post.

Assistant Professor in Structural Biology and Biochemistry, Department of Molecular Biology & Biochemistry
University of California, Irvine is hiring. Apply now!
recruit.ap.uci.edu
October 17, 2025 at 11:23 PM
Our home department of Molecular Biology & Biochemistry at UC Irvine is looking to hire a new tenure-track assistant professor in the broad area of structural biology. Come be our colleague! recruit.ap.uci.edu/JPF09887
Please apply and/or share this post.
Please apply and/or share this post.
Congratulations to Bryan Neumann from our collaborator Shane Gonen’s lab on receiving the Barbara K. Burgess Postdoctoral Fellowship Award — a well-deserved honor!
@gonenshane.bsky.social @ribbehulab.bsky.social @ucibiosci.bsky.social
@gonenshane.bsky.social @ribbehulab.bsky.social @ucibiosci.bsky.social

June 12, 2025 at 6:17 PM
Congratulations to Bryan Neumann from our collaborator Shane Gonen’s lab on receiving the Barbara K. Burgess Postdoctoral Fellowship Award — a well-deserved honor!
@gonenshane.bsky.social @ribbehulab.bsky.social @ucibiosci.bsky.social
@gonenshane.bsky.social @ribbehulab.bsky.social @ucibiosci.bsky.social
Our second paper that came online within a week 😊! Thanks everyone for their hard work!
@ribbehulab.bsky.social @ucibiosci.bsky.social
Heterologous synthesis of a simplified nitrogenase analog in Escherichia coli | Science Advances www.science.org/doi/10.1126/...
@ribbehulab.bsky.social @ucibiosci.bsky.social
Heterologous synthesis of a simplified nitrogenase analog in Escherichia coli | Science Advances www.science.org/doi/10.1126/...
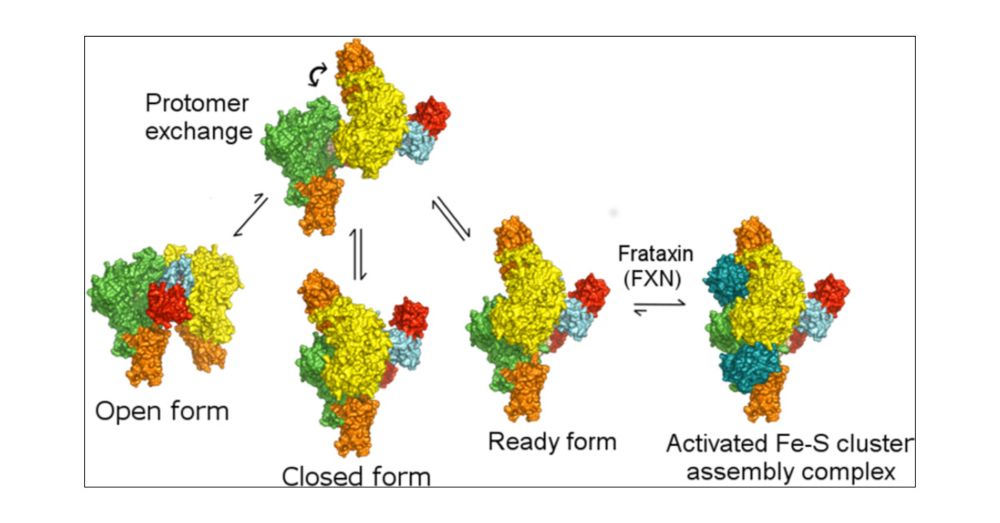
Heterologous synthesis of a simplified nitrogenase analog in Escherichia coli
Heterologous synthesis of a nitrogenase analog (NifH/NifEN) in E. coli enables N2 reduction and incorporation of N into biomass.
www.science.org
May 2, 2025 at 6:51 PM
Our second paper that came online within a week 😊! Thanks everyone for their hard work!
@ribbehulab.bsky.social @ucibiosci.bsky.social
Heterologous synthesis of a simplified nitrogenase analog in Escherichia coli | Science Advances www.science.org/doi/10.1126/...
@ribbehulab.bsky.social @ucibiosci.bsky.social
Heterologous synthesis of a simplified nitrogenase analog in Escherichia coli | Science Advances www.science.org/doi/10.1126/...
Reposted by Ribbe Hu Labs
Our story detailing how asymmetry in nitrogenase-like proteins regulate electron transfer reactions is finally out! rdcu.be/ejaeK Congratulations to postdoc @rajnandani.bsky.social and fantastic collaborators. Thanks to funding from the Department of Energy and the NIH.
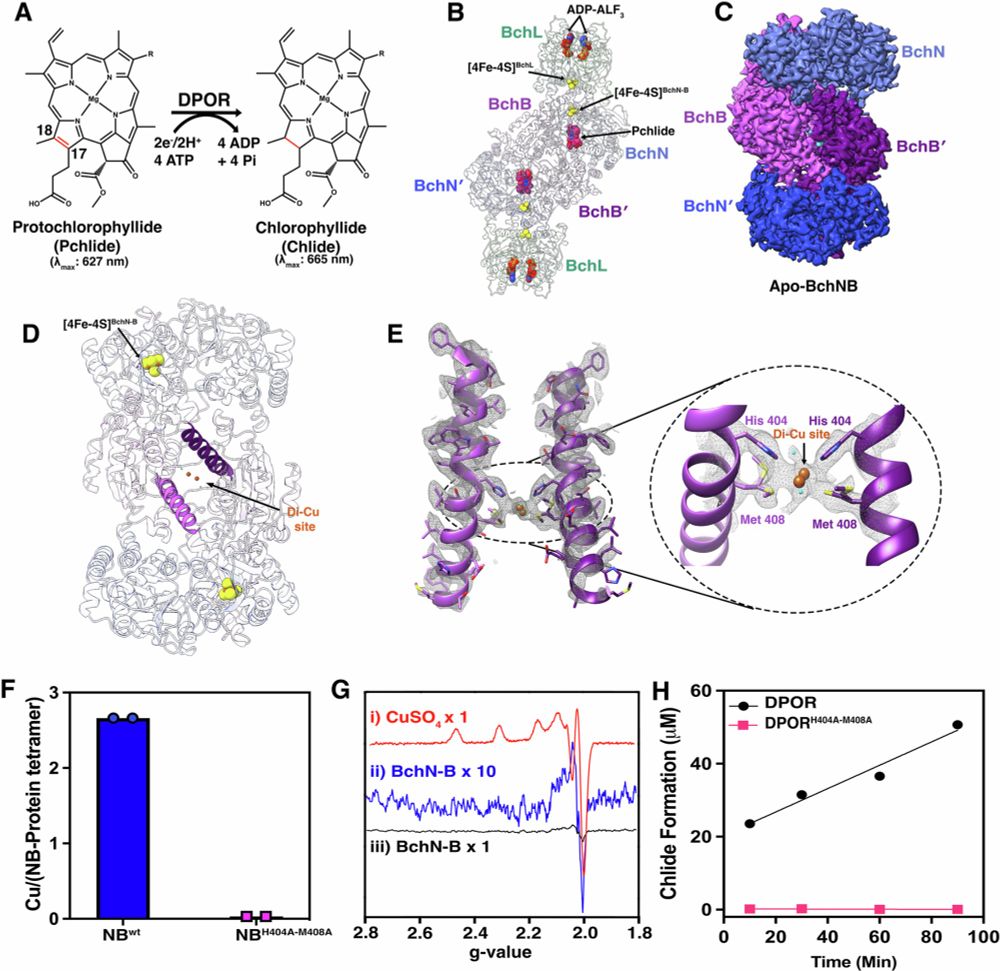
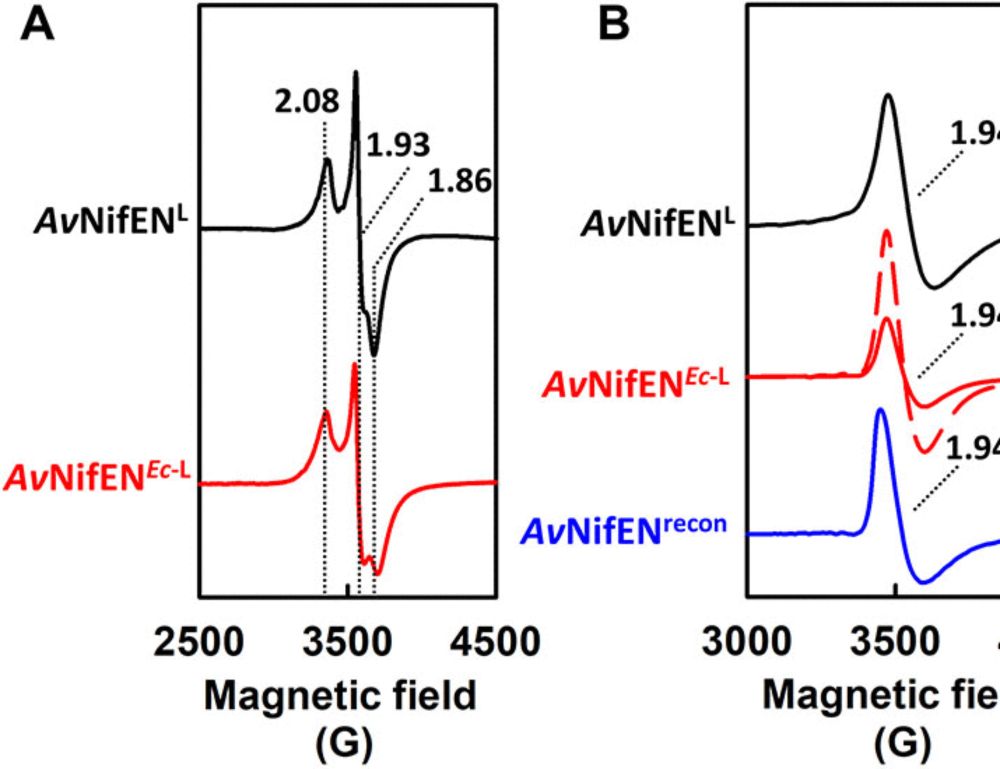
Cryo-EM captures the coordination of asymmetric electron transfer through a di-copper site in DPOR
Nature Communications - CryoEM snapshots of the nitrogenase-like DPOR protein complex captured during turnover reveal that asymmetric conformational changes, substrate recognition, and an interplay...
rdcu.be
April 24, 2025 at 2:11 PM
Our story detailing how asymmetry in nitrogenase-like proteins regulate electron transfer reactions is finally out! rdcu.be/ejaeK Congratulations to postdoc @rajnandani.bsky.social and fantastic collaborators. Thanks to funding from the Department of Energy and the NIH.
Excited to share our latest work highlighting the crucial role of belt-sulfur mobilization in nitrogenase catalysis. A big thank you to everyone who contributed to this effort!
@cp-chemcatalysis.bsky.social @ucibiosci.bsky.social
www.cell.com/chem-catalys...
@cp-chemcatalysis.bsky.social @ucibiosci.bsky.social
www.cell.com/chem-catalys...
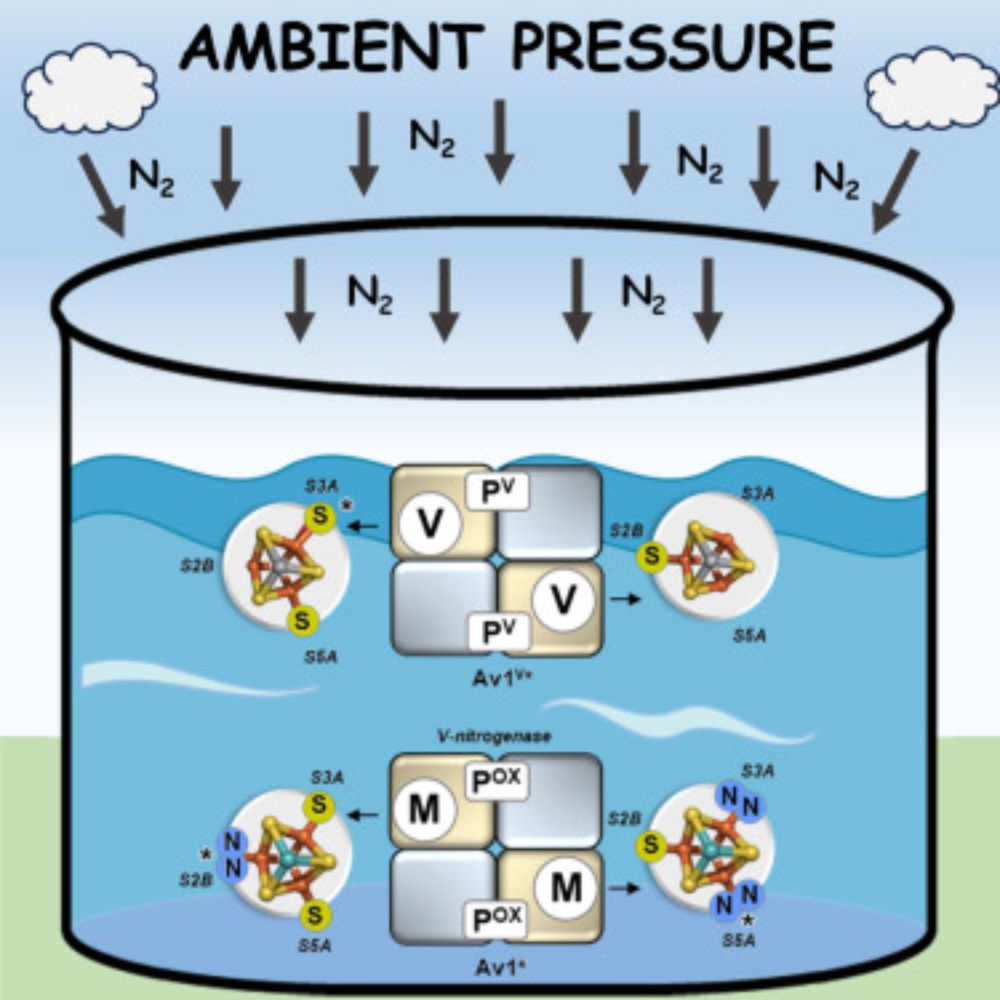
Belt-sulfur mobilization as a crucial mechanistic feature shared between the vanadium and molybdenum nitrogenases
Nitrogenase catalyzes the reduction of N2 to NH3 at its active site cofactor. Catalysis
by the homologous V- and Mo-nitrogenases involves the same dynamic belt-S mobilization
that occurs asymmetricall...
www.cell.com
April 28, 2025 at 4:21 PM
Excited to share our latest work highlighting the crucial role of belt-sulfur mobilization in nitrogenase catalysis. A big thank you to everyone who contributed to this effort!
@cp-chemcatalysis.bsky.social @ucibiosci.bsky.social
www.cell.com/chem-catalys...
@cp-chemcatalysis.bsky.social @ucibiosci.bsky.social
www.cell.com/chem-catalys...
Reposted by Ribbe Hu Labs
Our #CryoEM study on the binding of a Tarantula toxin to a full-length human voltage-gated sodium channel has been published. Very proud of the awesome people in my lab 🥳🥂
www.nature.com/articles/s41...
www.nature.com/articles/s41...
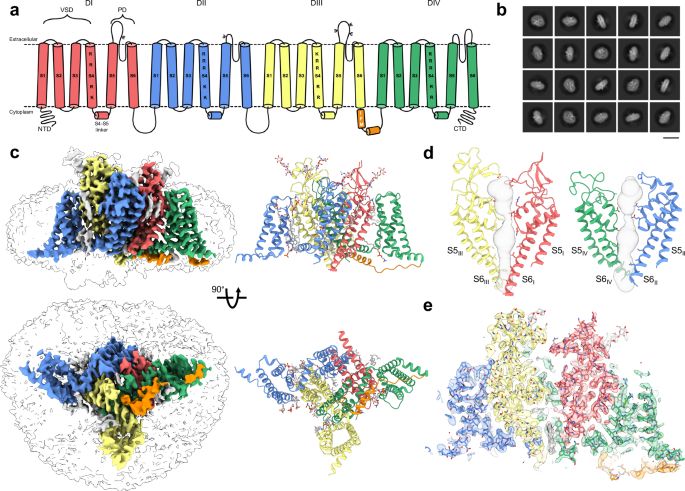
Structural basis of inhibition of human NaV1.8 by the tarantula venom peptide Protoxin-I - Nature Communications
Animal toxins can modulate action potentials and are important leads for therapeutics. Here, the authors use cryo-EM to show the interaction of the tarantula venom peptide Protoxin-I with a human volt...
www.nature.com
February 7, 2025 at 9:01 PM
Our #CryoEM study on the binding of a Tarantula toxin to a full-length human voltage-gated sodium channel has been published. Very proud of the awesome people in my lab 🥳🥂
www.nature.com/articles/s41...
www.nature.com/articles/s41...