Makoto T Hayashi
@makotothayashi.bsky.social
Group leader at IFOM-KU joint lab is working on #chromosome biology, especially focusing on #telomere functions. Employed by IFOM ETS and running the lab at Kyoto University.
10/10 For a more detailed explanation, check out bsky.app/profile/thec... for an in-depth thread on our findings.
1/Out today in @naturecomms.bsky.social,
“A CPC-shelterin-BTR axis regulates mitotic telomere deprotection”.
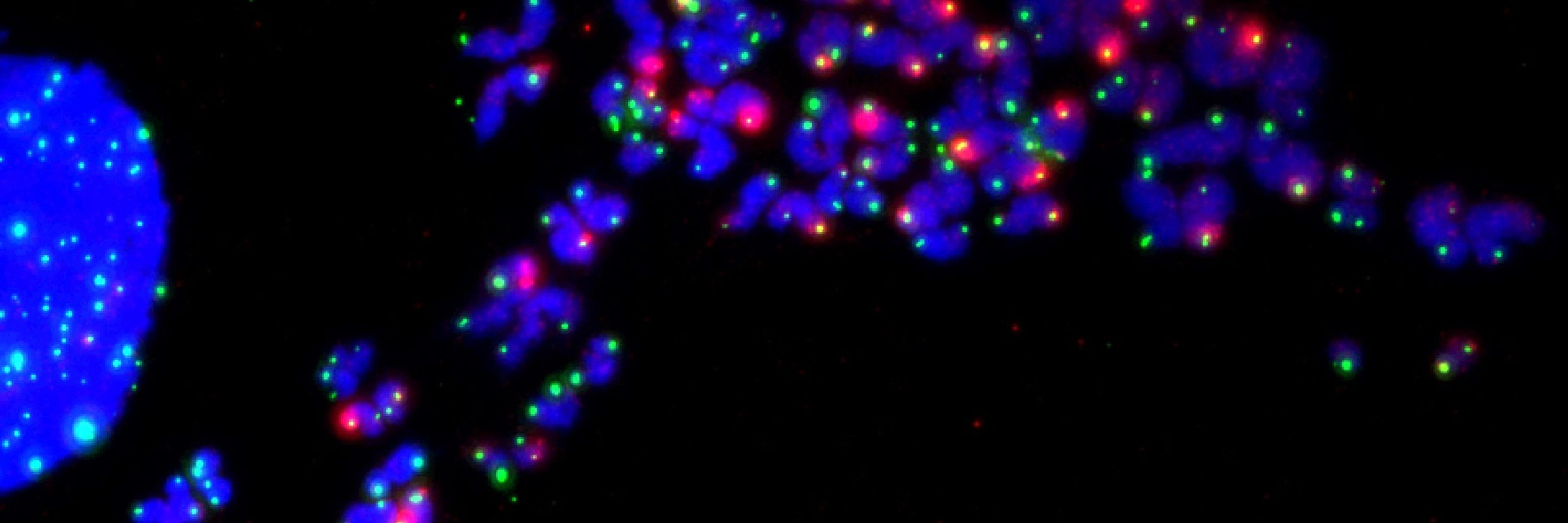
Here we identify the mechanism that unwinds telomere-loops (t-loops) during mitotic arrest to activate the DNA damage response and signal mitotic stress.
www.nature.com/articles/s41....
“A CPC-shelterin-BTR axis regulates mitotic telomere deprotection”.
Here we identify the mechanism that unwinds telomere-loops (t-loops) during mitotic arrest to activate the DNA damage response and signal mitotic stress.
www.nature.com/articles/s41....
A CPC-shelterin-BTR axis regulates mitotic telomere deprotection - Nature Communications
Here the authors reveal how telomeres signal mitotic stress. A key protein network alters their structure exposing telomere ends to signal mitotic stress, ultimately triggering a controlled DNA damage...
www.nature.com
March 17, 2025 at 12:49 PM
10/10 For a more detailed explanation, check out bsky.app/profile/thec... for an in-depth thread on our findings.
9/10 These insights not only advance our understanding of telomere biology but also opens potential therapeutic avenues for targeting mitotic death in cancer cells.
March 17, 2025 at 12:49 PM
9/10 These insights not only advance our understanding of telomere biology but also opens potential therapeutic avenues for targeting mitotic death in cancer cells.
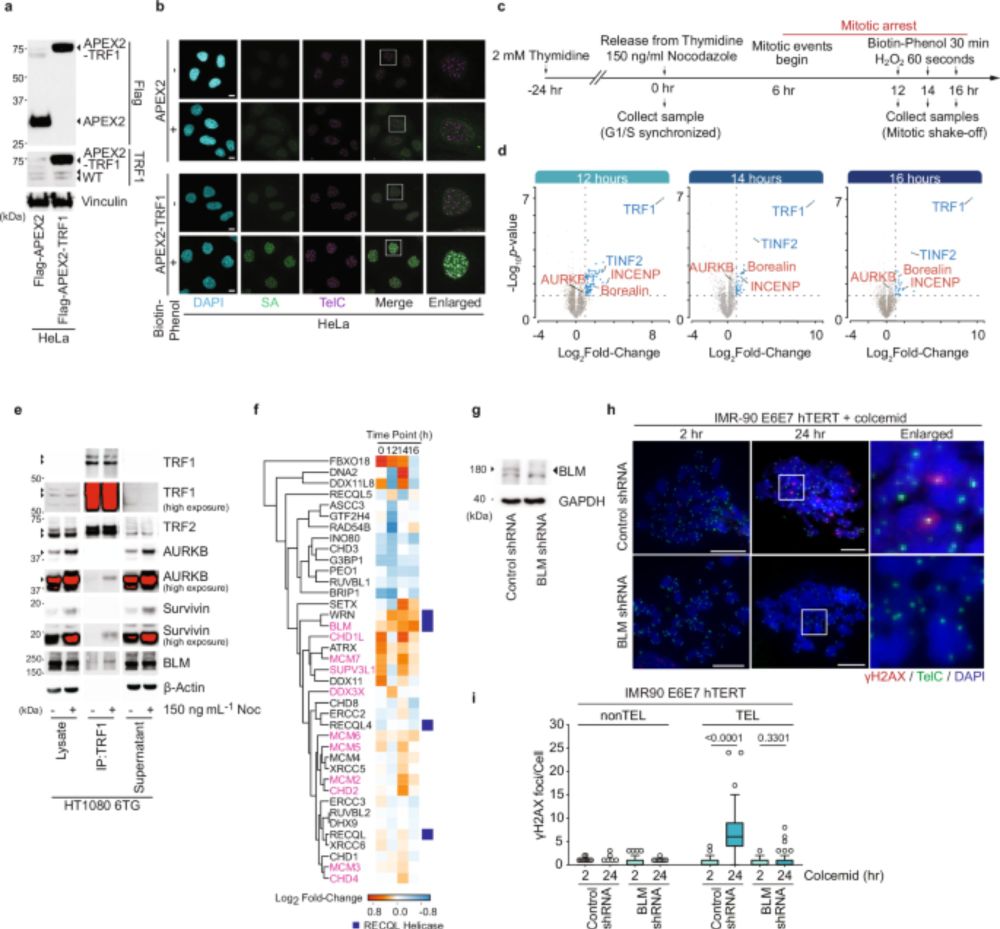
8/10 Our findings support a dynamic model where coordinated post-translational modifications of shelterin promote t-loop unwinding, aiding in the removal of damaged cells from the cell cycle.
March 17, 2025 at 12:49 PM
8/10 Our findings support a dynamic model where coordinated post-translational modifications of shelterin promote t-loop unwinding, aiding in the removal of damaged cells from the cell cycle.
7/10 This phosphorylation is essential for BTR-mediated double Holliday junction dissolution and contributes to mitotic telomere deprotection, highlighting a previously underappreciated role of the shelterin complex.
March 17, 2025 at 12:49 PM
7/10 This phosphorylation is essential for BTR-mediated double Holliday junction dissolution and contributes to mitotic telomere deprotection, highlighting a previously underappreciated role of the shelterin complex.
6/10 Shelterin is a telomere-binding protein complex that includes TRF1 and TRF2, crucial for maintaining telomere structure and function. During mitotic arrest, Aurora Kinase B (AURKB) of the CPC phosphorylates TRF1 and TRF2, leading to telomere linearity and a telomere-specific DDR.
March 17, 2025 at 12:49 PM
6/10 Shelterin is a telomere-binding protein complex that includes TRF1 and TRF2, crucial for maintaining telomere structure and function. During mitotic arrest, Aurora Kinase B (AURKB) of the CPC phosphorylates TRF1 and TRF2, leading to telomere linearity and a telomere-specific DDR.
5/10 Our study uncovers a sophisticated mechanism involving the Chromosome Passenger Complex (CPC), the shelterin complex, and the BLM-TOP3A-RMI1/2 (BTR) complex in regulating MAD-telomere deprotection.

March 17, 2025 at 12:49 PM
5/10 Our study uncovers a sophisticated mechanism involving the Chromosome Passenger Complex (CPC), the shelterin complex, and the BLM-TOP3A-RMI1/2 (BTR) complex in regulating MAD-telomere deprotection.
4/10 Telomeres safeguard chromosome ends by forming t-loops, preventing ATM activation and unwarranted DNA damage responses (DDR). However, during mitotic arrest, telomere linearity and a localized DDR emerge—a phenomenon known as "Mitotic Arrest Dependent (MAD)-Telomere Deprotection."

March 17, 2025 at 12:49 PM
4/10 Telomeres safeguard chromosome ends by forming t-loops, preventing ATM activation and unwarranted DNA damage responses (DDR). However, during mitotic arrest, telomere linearity and a localized DDR emerge—a phenomenon known as "Mitotic Arrest Dependent (MAD)-Telomere Deprotection."
3/10 Additional key contributors include Ronnie Low, Scott Page, Blake Lane, Andrew Robinson, and Lucy French from @thecesarelab.bsky.social. Fuyuki Ishikawa from Kyoto University and Shunya Kosaka from my lab.
March 17, 2025 at 12:49 PM
3/10 Additional key contributors include Ronnie Low, Scott Page, Blake Lane, Andrew Robinson, and Lucy French from @thecesarelab.bsky.social. Fuyuki Ishikawa from Kyoto University and Shunya Kosaka from my lab.
2/10 This work highlights the power of collaboration. Special thanks to our fantastic collaborator @thecesarelab.bsky.social. Diana Romero from my lab and Sam Rogers from Cesare’s lab contributed equally to this work. Their perseverance truly deserves immense recognition!
March 17, 2025 at 12:49 PM
2/10 This work highlights the power of collaboration. Special thanks to our fantastic collaborator @thecesarelab.bsky.social. Diana Romero from my lab and Sam Rogers from Cesare’s lab contributed equally to this work. Their perseverance truly deserves immense recognition!

