MSc SynCat Wolf Group Regensburg 🐺| Catalysis |Bioinorganic Chemistry |Inorganic Synthesis |
🇧🇦
(he/him)

pubs.acs.org/doi/10.1021/...
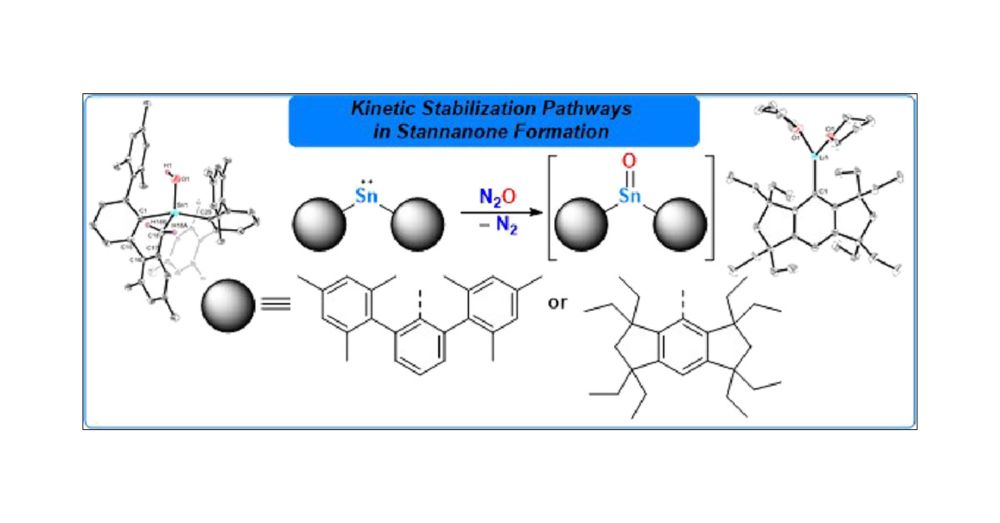
pubs.acs.org/doi/10.1021/...

This work, led by the exceptional @bjoernpfund.bsky.social, offers new insights into photochemical reactivity.
Published in JACS @jacs.acspublications.org
bit.ly/3IysmjX
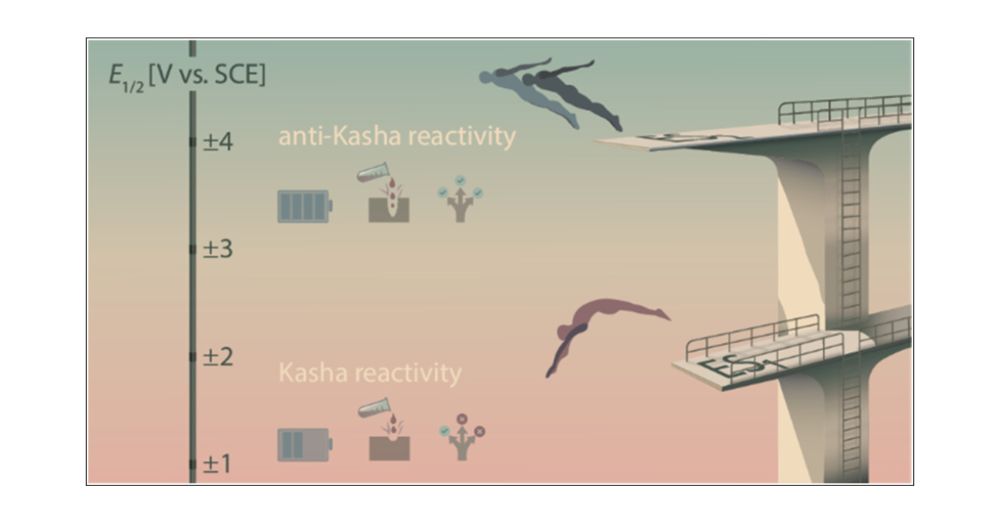
This work, led by the exceptional @bjoernpfund.bsky.social, offers new insights into photochemical reactivity.
Published in JACS @jacs.acspublications.org
bit.ly/3IysmjX

With Giacomo Morselli and Christian Reber in JACS @jacs.acspublications.org
pubs.acs.org/doi/10.1021/...
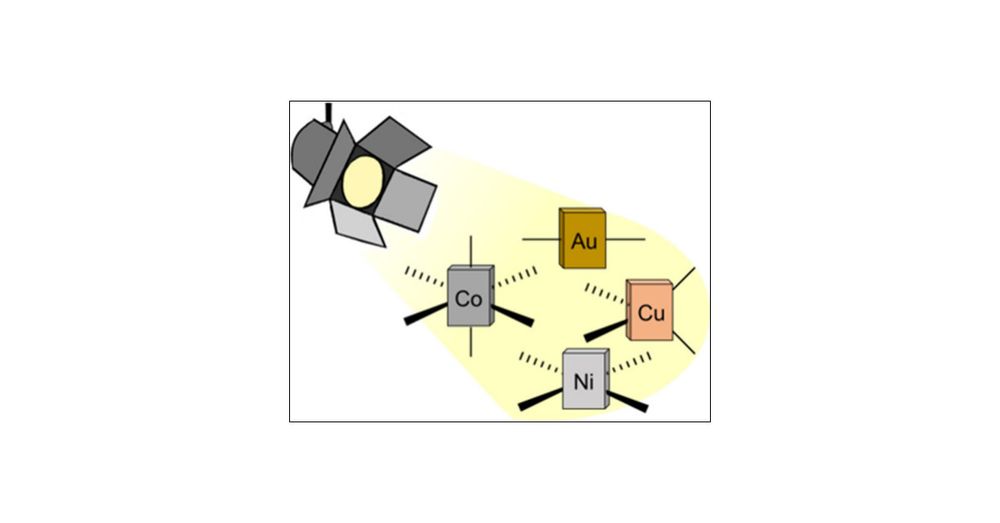
With Giacomo Morselli and Christian Reber in JACS @jacs.acspublications.org
pubs.acs.org/doi/10.1021/...
Thanks to @leverhulme.bsky.social we will soon be recruiting a new PDRA to study some unusual redox-active Lewis acid chemistry.
Full advert to follow soon, but in the meantime please feel free to get in touch if interested!
Thanks to @leverhulme.bsky.social we will soon be recruiting a new PDRA to study some unusual redox-active Lewis acid chemistry.
Full advert to follow soon, but in the meantime please feel free to get in touch if interested!
www.nature.com/articles/s41...
#chemsky
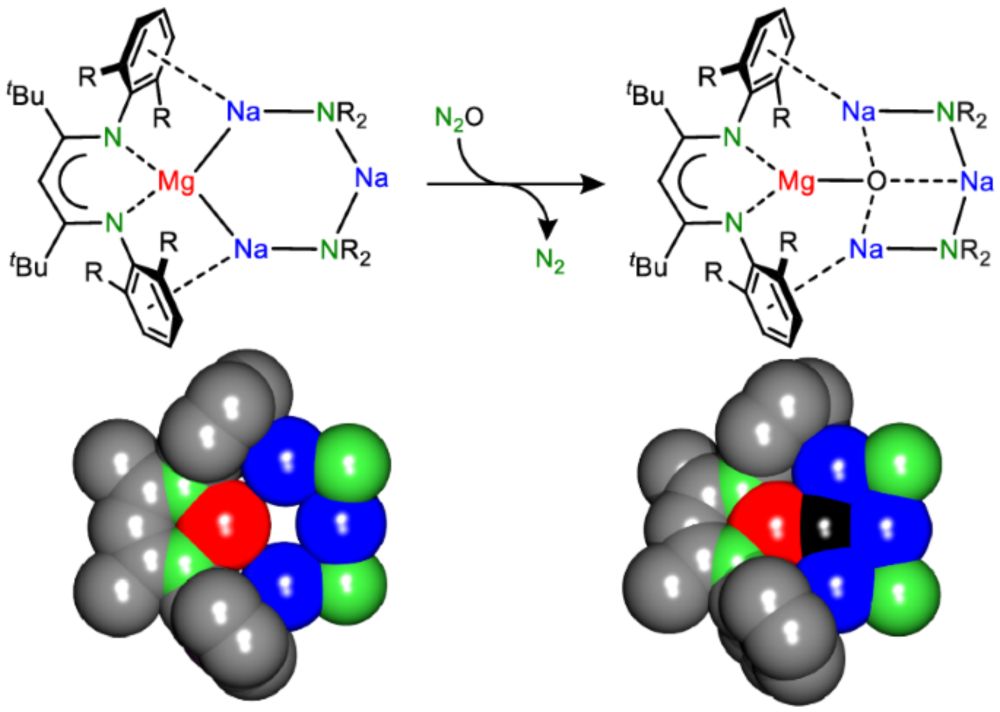
www.nature.com/articles/s41...
#chemsky
#BlackinChem cen.acs.org/careers/dive...

#BlackinChem cen.acs.org/careers/dive...
#WomenInScience #IDWGS

#WomenInScience #IDWGS
www.nature.com/articles/s41...
#chemsky

www.nature.com/articles/s41...
#chemsky




