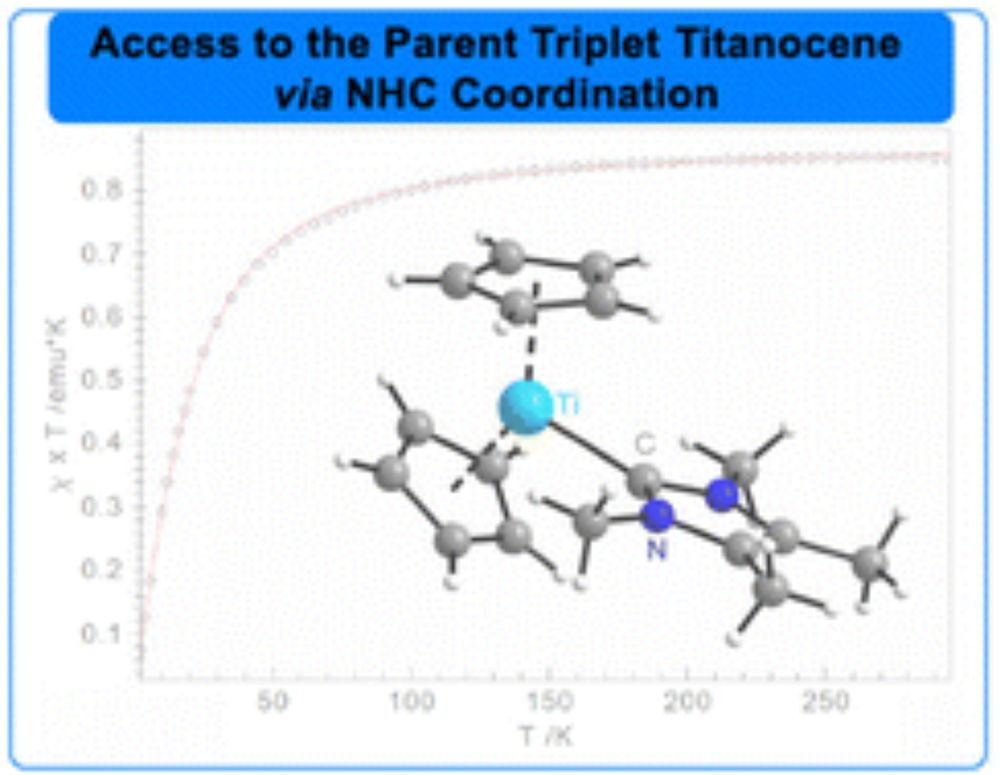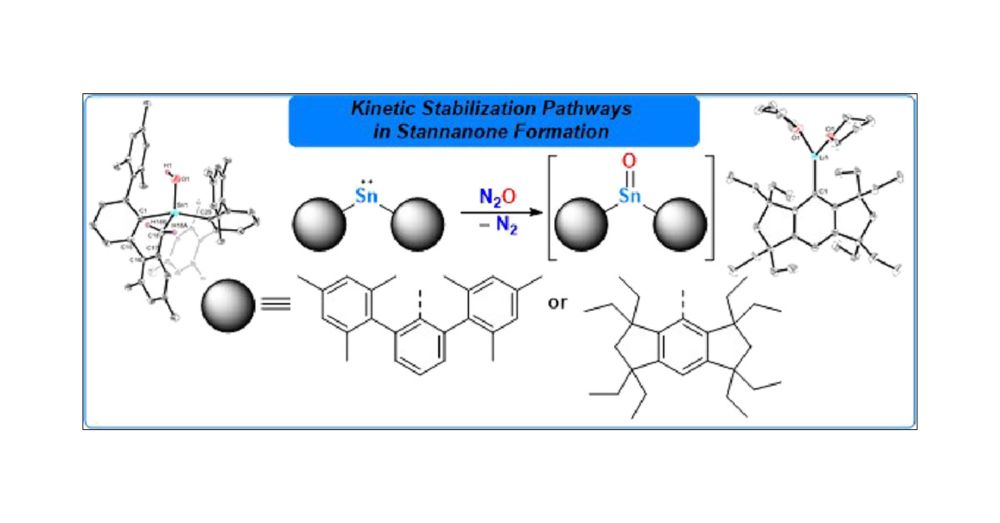
pubs.acs.org/doi/10.1021/...
My group at @manchester.ac.uk is recruiting a 3.5-year PhD student to explore Sb & Bi pincer complexes for metallomimetic chemistry.
🔬 Fully funded (UK only)
📍 Manchester
🧪 Synthetic inorganic chemistry
Apply 👉 tinyurl.com/yrjkvha9


Awesome work by Soti and a great cooperation with the Bittl group and @franemmerling.bsky.social.
Dedicated to @christianlimberg.bsky.social and Franc Meyer
@humboldtuni.bsky.social @manchester.ac.uk
onlinelibrary.wiley.com/doi/full/10....

Awesome work by Soti and a great cooperation with the Bittl group and @franemmerling.bsky.social.
Dedicated to @christianlimberg.bsky.social and Franc Meyer
@humboldtuni.bsky.social @manchester.ac.uk
onlinelibrary.wiley.com/doi/full/10....
I’m over the moon to share the first publication from our small research group! 🚀 pubs.rsc.org/en/content/a... @chemcomm.rsc.org
Jan explored a funky P=P-containing heterocycle and its reversible! bond activation chemistry, with DFT insights from Francesco. #newPI
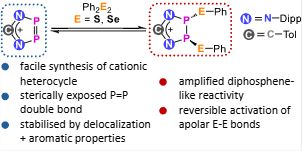
I’m over the moon to share the first publication from our small research group! 🚀 pubs.rsc.org/en/content/a... @chemcomm.rsc.org
Jan explored a funky P=P-containing heterocycle and its reversible! bond activation chemistry, with DFT insights from Francesco. #newPI
Thanks a lot to @chemieverband.bsky.social for the generous funding. pubs.acs.org/doi/10.1021/...

Thanks a lot to @chemieverband.bsky.social for the generous funding. pubs.acs.org/doi/10.1021/...


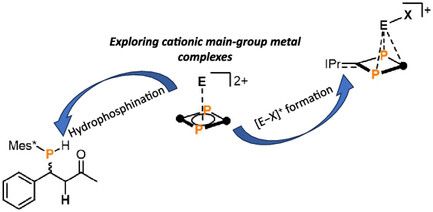
Philipp elucidated the mechanism of the dual #emission, the #excited state #dynamics, #excimer formation and light-induced bond #homolysis. #Maingroup #tin #photophysics #photochemistry onlinelibrary.wiley.com/doi/10.1002/anie.202510044
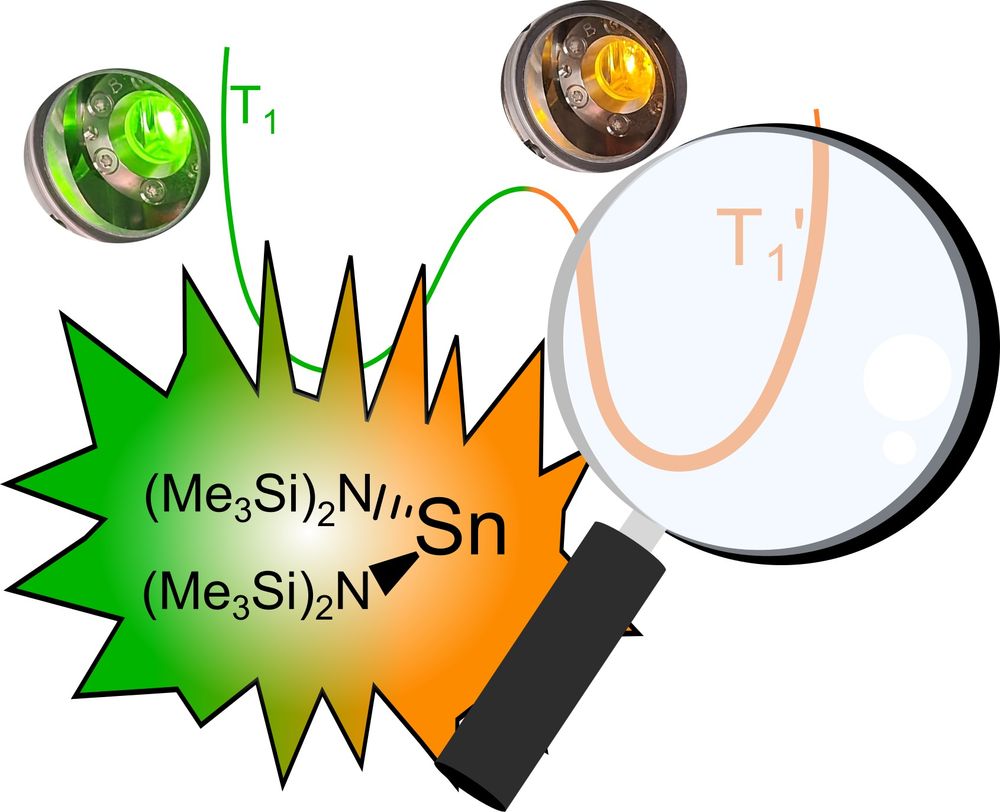
Philipp elucidated the mechanism of the dual #emission, the #excited state #dynamics, #excimer formation and light-induced bond #homolysis. #Maingroup #tin #photophysics #photochemistry onlinelibrary.wiley.com/doi/10.1002/anie.202510044
pubs.acs.org/doi/10.1021/...

pubs.acs.org/doi/10.1021/...

Hydrocarbon-soluble Mg(0) reduces Si all the way to Si(4-). Unfortunately not stable and decomposing to the HSi(3-) anion. However, Sn(4-) is stable and functions as 4-fold Nu or 8e reducing agent. Preprint: shorturl.at/4mH0O
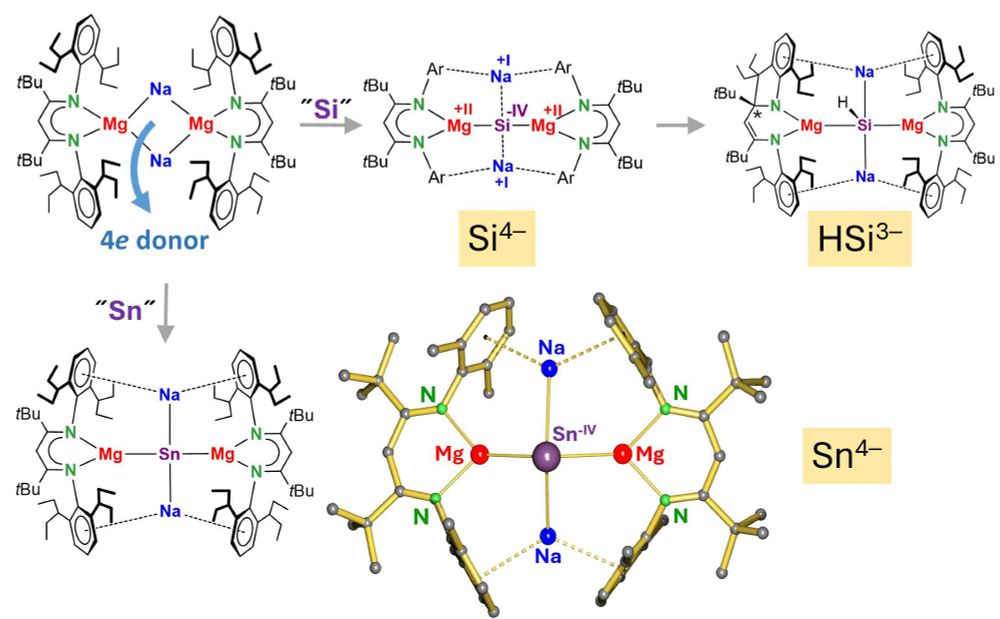
Hydrocarbon-soluble Mg(0) reduces Si all the way to Si(4-). Unfortunately not stable and decomposing to the HSi(3-) anion. However, Sn(4-) is stable and functions as 4-fold Nu or 8e reducing agent. Preprint: shorturl.at/4mH0O
pubs.rsc.org/en/content/a...
📍@unigoettingen.bsky.social and @kit.edu
🧪
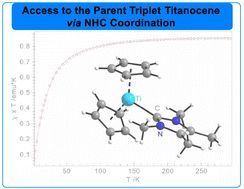
pubs.rsc.org/en/content/a...
📍@unigoettingen.bsky.social and @kit.edu
🧪
doi.org/10.1002/anie...
doi.org/10.1002/anie...
This collection represents the top 10% of research published in our journal each quarter. Congratulations to all the authors whose articles are featured in this collection👏
pubs.rsc.org/en/journals/...
#Chemsky 🧪

This collection represents the top 10% of research published in our journal each quarter. Congratulations to all the authors whose articles are featured in this collection👏
pubs.rsc.org/en/journals/...
#Chemsky 🧪
pubs.acs.org/doi/full/10....
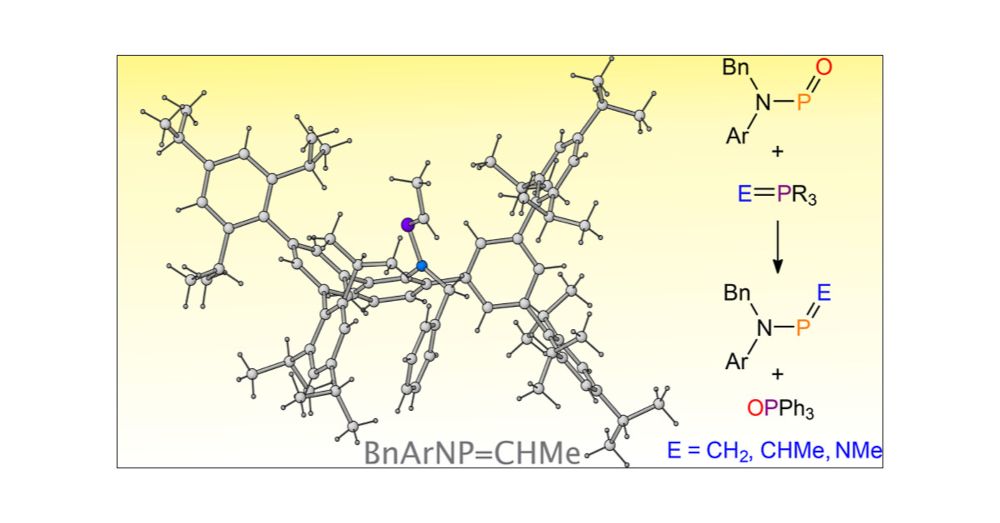
pubs.acs.org/doi/full/10....
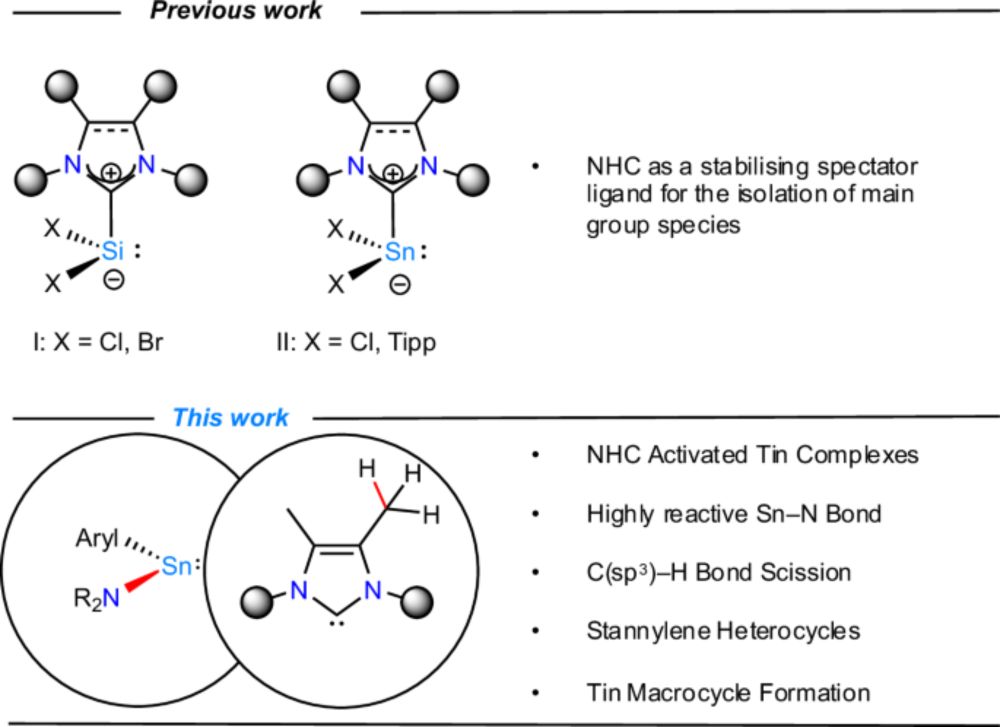
pubs.acs.org/doi/10.1021/...
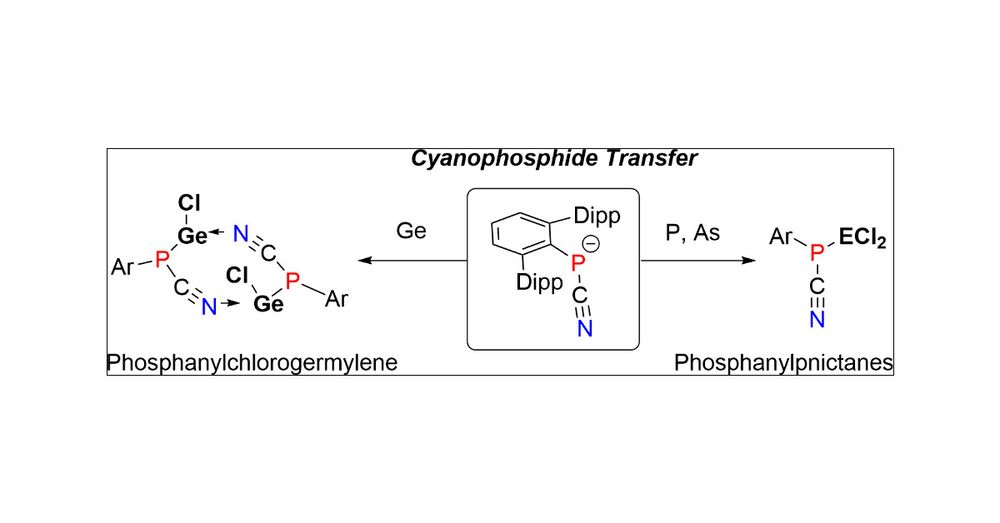
pubs.acs.org/doi/10.1021/...
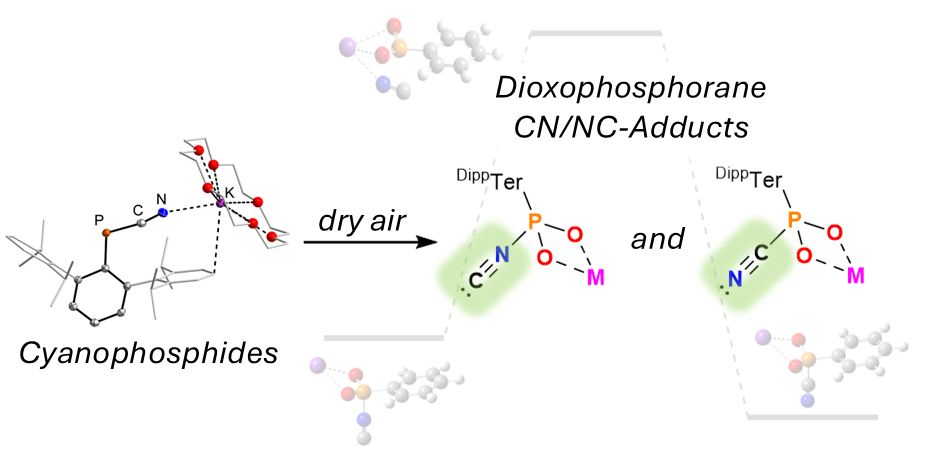
pubs.rsc.org/en/content/a...
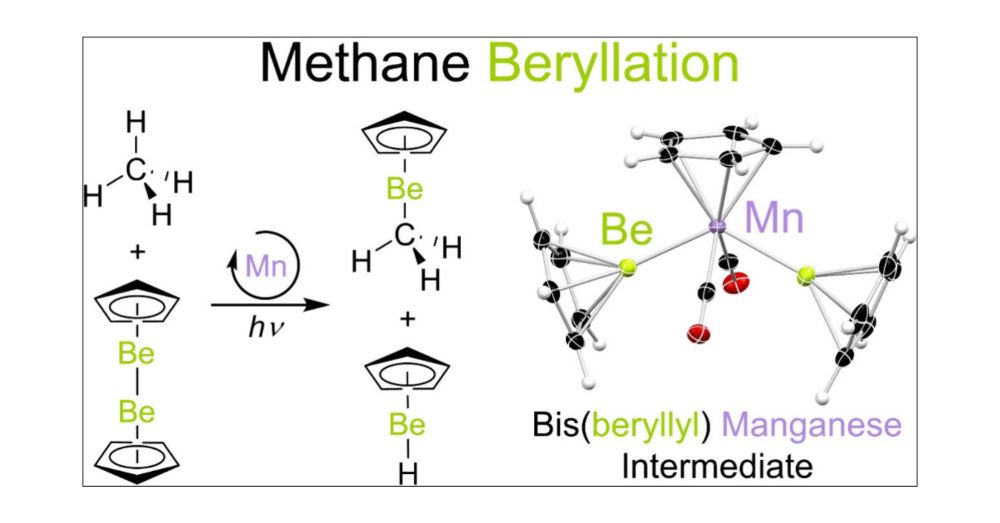
First up @angewandtechemie.bsky.social
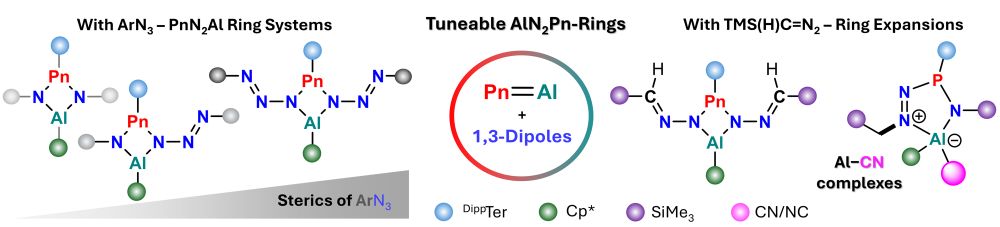
Macro/molecular chemistry of cis/trans P2N4 rings.
Congrats @michaelland.bsky.social w/ @jasonmasuda.bsky.social for Xray
onlinelibrary.wiley.com/doi/10.1002/...
cc @dalchemistry.bsky.social
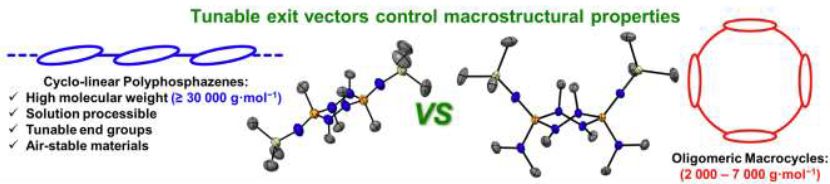
First up @angewandtechemie.bsky.social
Macro/molecular chemistry of cis/trans P2N4 rings.
Congrats @michaelland.bsky.social w/ @jasonmasuda.bsky.social for Xray
onlinelibrary.wiley.com/doi/10.1002/...
cc @dalchemistry.bsky.social