
https://luxton.faculty.ucdavis.edu
We call this the "nucleolar-ribosomal axis" and it explains the widespread cellular defects in EDMD. (4/6)

We call this the "nucleolar-ribosomal axis" and it explains the widespread cellular defects in EDMD. (4/6)

















Image

Image
This preprint is just the start--our future work explores how tissues maintain this balance during development and when responding to challenges.

This preprint is just the start--our future work explores how tissues maintain this balance during development and when responding to challenges.
This balance allows tissues to function, adapt to stress, and may even help us understand how diseases like cancer disrupt cellular order.
This balance allows tissues to function, adapt to stress, and may even help us understand how diseases like cancer disrupt cellular order.
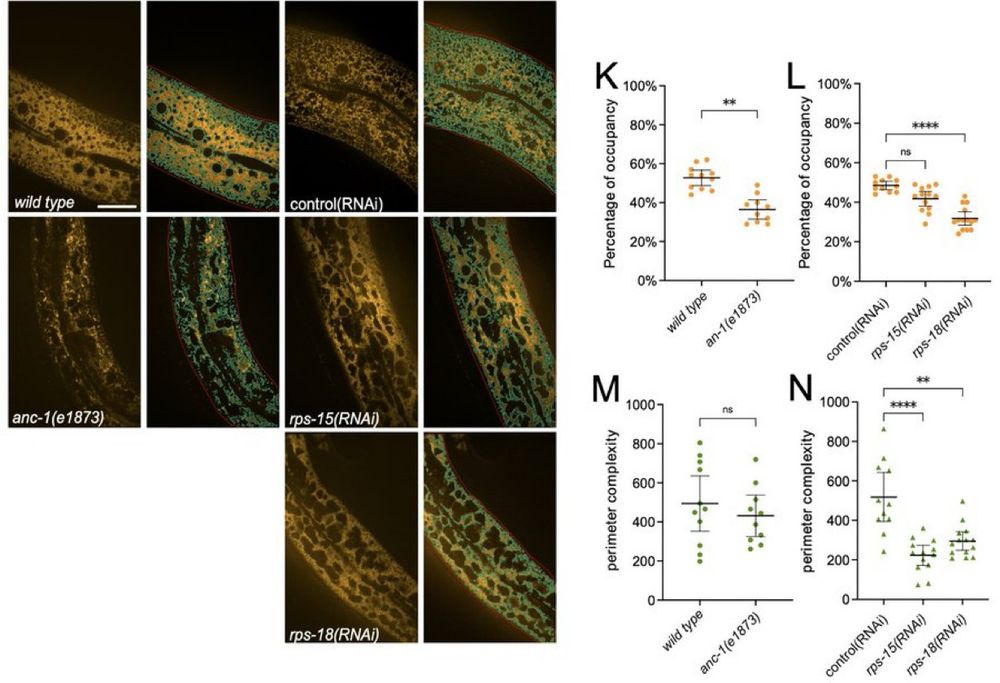
Ribosome depletion made the cytoplasm less crowded overall.
When both were disrupted, molecular movement increased dramatically.
This shows how these systems work together.

Ribosome depletion made the cytoplasm less crowded overall.
When both were disrupted, molecular movement increased dramatically.
This shows how these systems work together.
Ribosomes, best known as the cell's protein factories, also contribute to the crowded environment inside cells.
A giant protein called ANC-1 interacts with the endoplasmic reticulum (ER) to act as scaffolding, imposing structural constraints.

Ribosomes, best known as the cell's protein factories, also contribute to the crowded environment inside cells.
A giant protein called ANC-1 interacts with the endoplasmic reticulum (ER) to act as scaffolding, imposing structural constraints.

