
🧠 🐁 Functional Neuroimaging, Brain (dys)connectivity and Autism
ERC Grantee
www.gozzilab.it
19/n

19/n
17/n

17/n
15/n

15/n
13/n

13/n
⚡Even more striking: we found an EEG “Hurst exponent” signature that mirrors what’s seen in autistic individuals
12/n

⚡Even more striking: we found an EEG “Hurst exponent” signature that mirrors what’s seen in autistic individuals
12/n
11/n
11/n
This suggests that what we investigate here could be directly relevant to autism!
10/n

This suggests that what we investigate here could be directly relevant to autism!
10/n
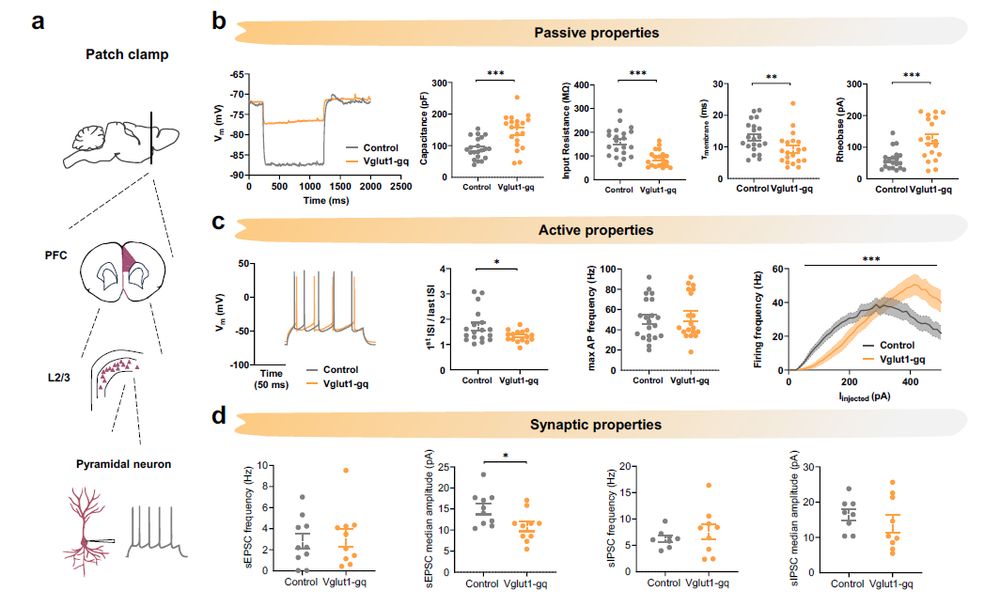
If so, manipulated mice should show a signature of transcriptional reprogramming. We thus tested this using RNAseq in cortex of manipulated mice
9/n

If so, manipulated mice should show a signature of transcriptional reprogramming. We thus tested this using RNAseq in cortex of manipulated mice
9/n
But what could be driving these social deficits?
8/n

But what could be driving these social deficits?
8/n
7/n

7/n
Control studies showed that this manipulation transiently increases neuronal excitability (without causing epilepsy). So far so good!
6/n

Control studies showed that this manipulation transiently increases neuronal excitability (without causing epilepsy). So far so good!
6/n
a short-lived imbalance during a critical early window might permanently derail circuit formation and produce autism-relevant phenotypes.
And this is precisely the hypothesis we set out to test in mice!
5/n

a short-lived imbalance during a critical early window might permanently derail circuit formation and produce autism-relevant phenotypes.
And this is precisely the hypothesis we set out to test in mice!
5/n
2/n

2/n
Here we tackle a long-standing chicken-or-egg 🐣🥚question in #autism and developmental neuroscience
➡️ Is excitation–inhibition (E:I) imbalance a "cause" or a "consequence" of #autism?
Check out what we found!
🧵1/n

The skull has long been a barrier to ultrasound. This reprint shows how to make it quickly transparent → enabling full-depth, high-res fUSI in mice (& humans! 🤯🤯)
Huge congrats to the authors!!!
www.biorxiv.org/content/10.1...

The skull has long been a barrier to ultrasound. This reprint shows how to make it quickly transparent → enabling full-depth, high-res fUSI in mice (& humans! 🤯🤯)
Huge congrats to the authors!!!
www.biorxiv.org/content/10.1...
Deeply grateful to this outstanding community for its openness, curiosity & for welcoming outsiders like me with such warmth and generosity💙
I’ll carry the memories of this experience with me for a long, long time!



Deeply grateful to this outstanding community for its openness, curiosity & for welcoming outsiders like me with such warmth and generosity💙
I’ll carry the memories of this experience with me for a long, long time!
See you all in Brisbane!
See you all in Brisbane!
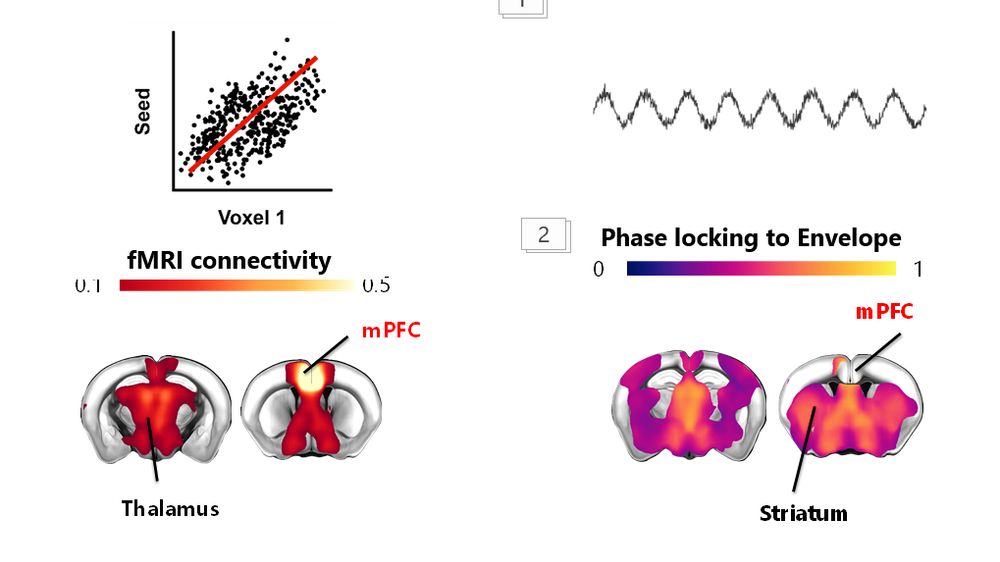
1️⃣ In poster presentation #1311, we will show how a brief and transient developmental Excitation/Inhibition imbalance may irreversibly disrupt cortical fMRI connectivity. How? Epigenetics is key!

1️⃣ In poster presentation #1311, we will show how a brief and transient developmental Excitation/Inhibition imbalance may irreversibly disrupt cortical fMRI connectivity. How? Epigenetics is key!
📄https://tinyurl.com/42spkbft
Here we outline key challenges & a roadmap to advance the field! Proud of this collaborative effort 🥳🎉

📄https://tinyurl.com/42spkbft
Here we outline key challenges & a roadmap to advance the field! Proud of this collaborative effort 🥳🎉
1️⃣ 🧠 Watch the 🐭 brain in motion, with functional ultrasound imaging (fUSI)
2️⃣🧪 Mess with the 🐭 brain (experimentally) via causal perturbations + imaging
🔬 Neuro/physics/bioengeneering backgrounds welcome
👉Info ▶️https://lnkd.in/dDeTTDj2

1️⃣ 🧠 Watch the 🐭 brain in motion, with functional ultrasound imaging (fUSI)
2️⃣🧪 Mess with the 🐭 brain (experimentally) via causal perturbations + imaging
🔬 Neuro/physics/bioengeneering backgrounds welcome
👉Info ▶️https://lnkd.in/dDeTTDj2
15/n

15/n
We thus hypothesized that this phenomenon could be associated with autism-relevant symptoms
14/n

We thus hypothesized that this phenomenon could be associated with autism-relevant symptoms
14/n
13/n

13/n
To test this, we used gene decoding analyses to investigate if brain regions exhibiting developmental dysconnectivity would be specifically enriched for synaptic-relevant GSK3β‐interactors
12/n

To test this, we used gene decoding analyses to investigate if brain regions exhibiting developmental dysconnectivity would be specifically enriched for synaptic-relevant GSK3β‐interactors
12/n
11/n

11/n

