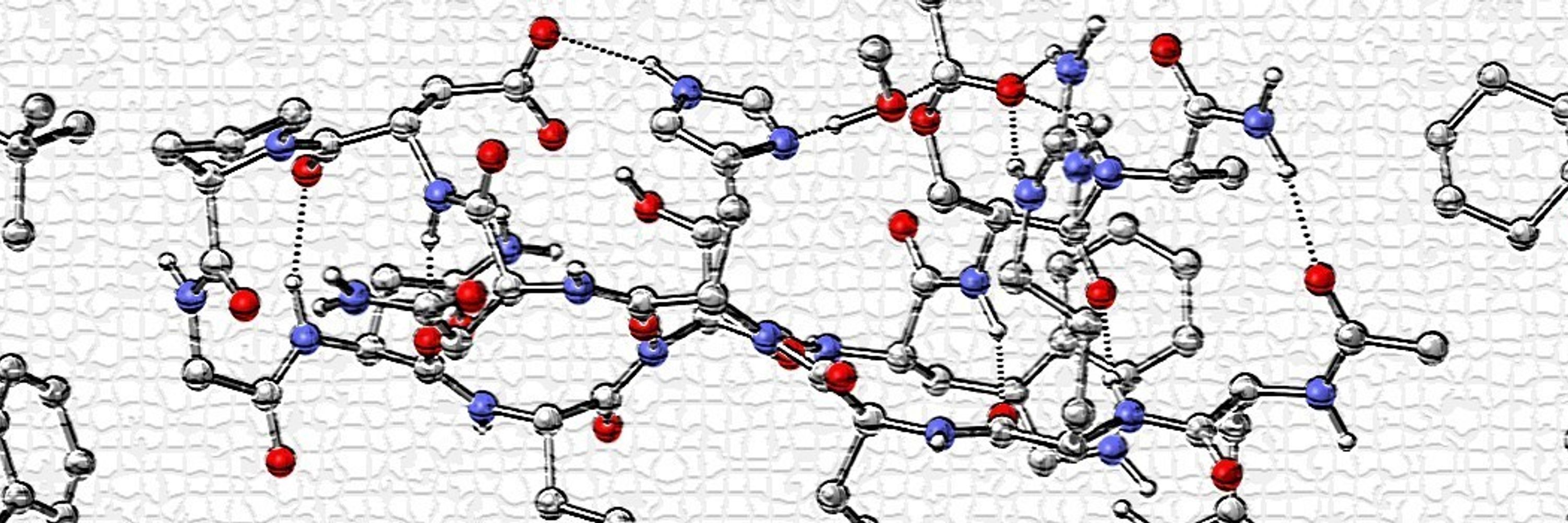
www.nature.com/articles/s41...
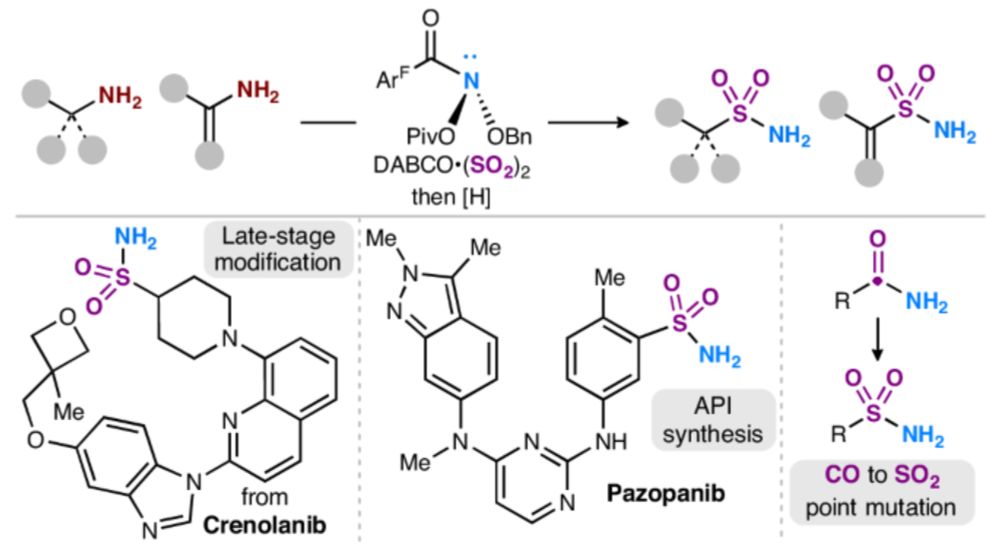
www.nature.com/articles/s41...

pubs.acs.org/doi/full/10....
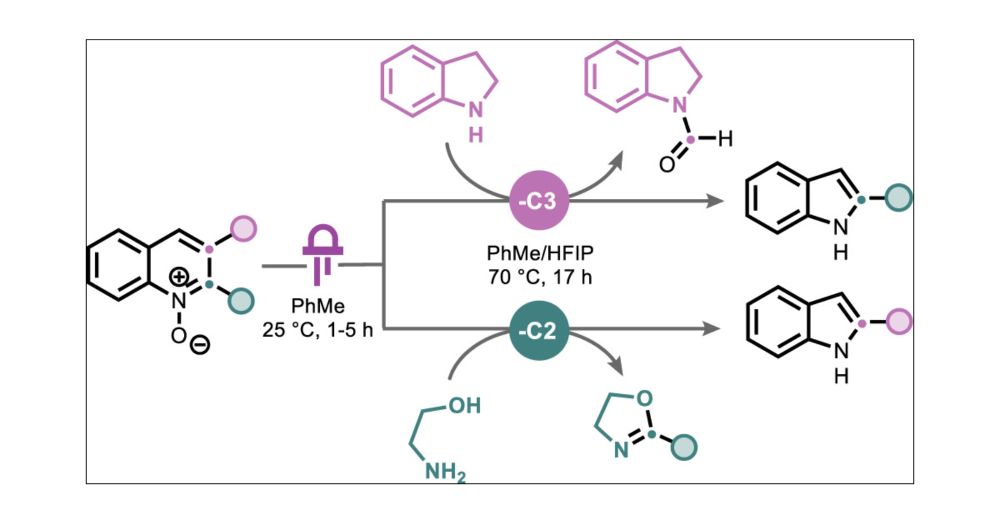
pubs.acs.org/doi/full/10....
A solution to the pyrazole alkylation problem, leveraging S-to-NR atom replacement. Skeletal editing has strategic value, outside of late-stage!
www.nature.com/articles/s41...
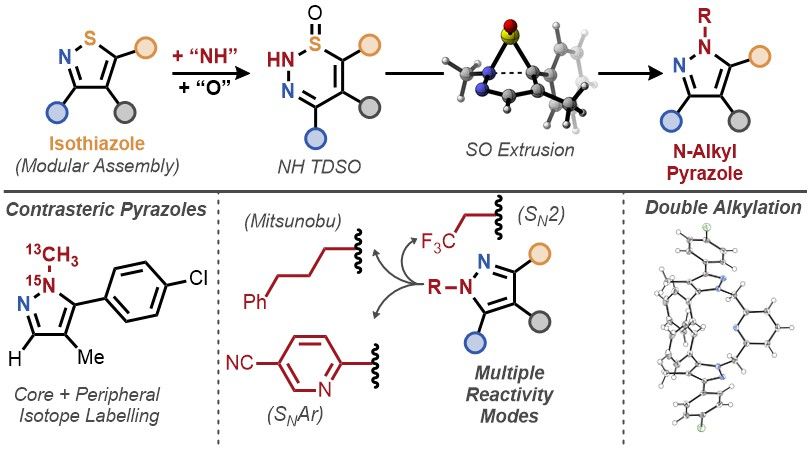
A solution to the pyrazole alkylation problem, leveraging S-to-NR atom replacement. Skeletal editing has strategic value, outside of late-stage!
www.nature.com/articles/s41...
tinyurl.com/42re37pd

tinyurl.com/42re37pd
coordinated with the Leonori group onlinelibrary.wiley.com/doi/10.1002/...

coordinated with the Leonori group onlinelibrary.wiley.com/doi/10.1002/...
chemrxiv.org/engage/chemr...
chemrxiv.org/engage/chemr...

chemrxiv.org/engage/chemr...
chemrxiv.org/engage/chemr...
Photochem-mediated oxygen migration into sp3 bonds delivering small ring ethers as well as an OH - methylene transposition
#ChemSky 1/
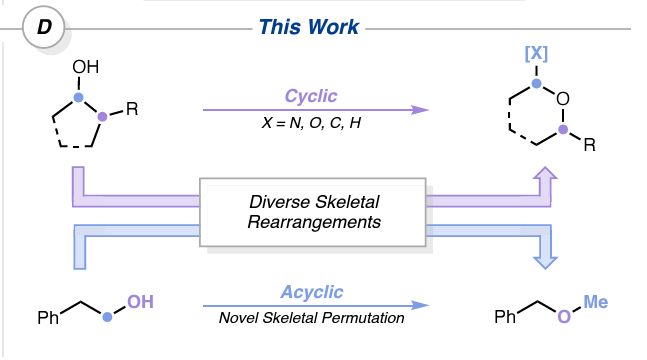
Photochem-mediated oxygen migration into sp3 bonds delivering small ring ethers as well as an OH - methylene transposition
#ChemSky 1/
pubs.acs.org/doi/10.1021/...

pubs.acs.org/doi/10.1021/...
doi.org/10.1021/jacs...

doi.org/10.1021/jacs...
What happens when unconventional ideas drive to push boundaries? 🚀 Meet FerroTACs: a novel #PROTAC design using ferrocene as core unit to re-imagining linker design and fine-tune their phys-chem properties. Learn more about our work led by @alesalerno.bsky.social here: 🧪👇 1/5

What happens when unconventional ideas drive to push boundaries? 🚀 Meet FerroTACs: a novel #PROTAC design using ferrocene as core unit to re-imagining linker design and fine-tune their phys-chem properties. Learn more about our work led by @alesalerno.bsky.social here: 🧪👇 1/5


Apply by Jan 17: survey.sogolytics.com/survey1.aspx...
Contact us at chemistsofcolor@merck.com #ChemSky

Apply by Jan 17: survey.sogolytics.com/survey1.aspx...
Contact us at chemistsofcolor@merck.com #ChemSky


pubs.rsc.org/en/content/a...
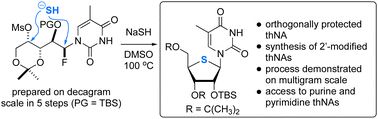
pubs.rsc.org/en/content/a...




chemrxiv.org/engage/chemr...
Very useful analysis - should spur lots more development in skeletal editing!
@charlesthechemist.bsky.social (Looks like Matt and Richmond aren't here yet?)
chemrxiv.org/engage/chemr...
Very useful analysis - should spur lots more development in skeletal editing!
@charlesthechemist.bsky.social (Looks like Matt and Richmond aren't here yet?)
Air stable Ir precat for C-H borylation with large scope and 1-pot Borylation-Suzuki, bromination or Chan-Lam
Super paper and will be of high use in med chem #ChemSky
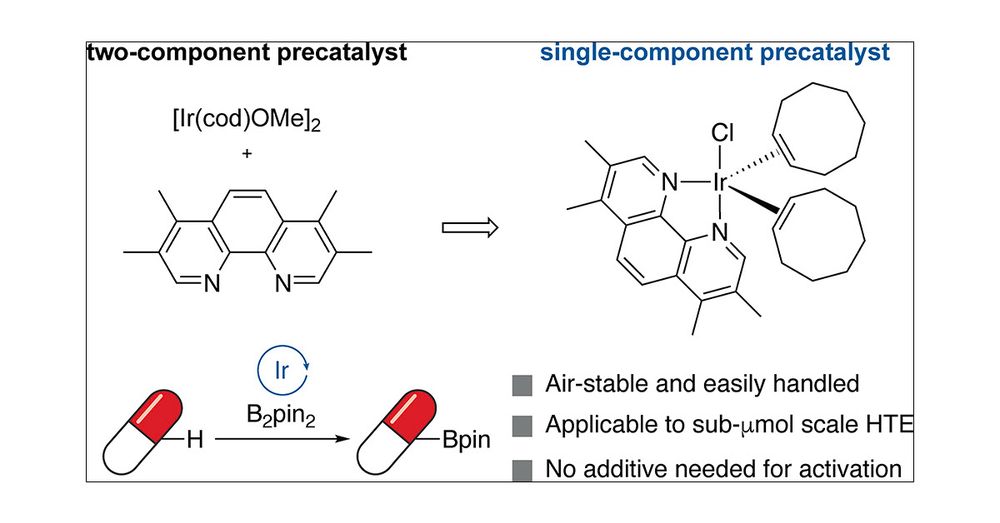
Air stable Ir precat for C-H borylation with large scope and 1-pot Borylation-Suzuki, bromination or Chan-Lam
Super paper and will be of high use in med chem #ChemSky

