


I'll always cherish our lovely times in NYC, Singapore, Zurich, and Sydney. My heartfelt condolences to his family and friends in this difficult time.
So long, Mike 🥺😔

We are thrilled to announce our amazing list of keynote speakers, who will help us to make this conference a major event in mechanobiology!

We are thrilled to announce our amazing list of keynote speakers, who will help us to make this conference a major event in mechanobiology!
@jorgealmagro.bsky.social, @thefuchslab.bsky.social et al. discuss the role of stem cell mechanics during developmental processes and what happens when the mechanics of the stem cell niche are disrupted in cancer.
👉 go.nature.com/3EsJzJx
#StemCells #Mechanobiology #Cancer
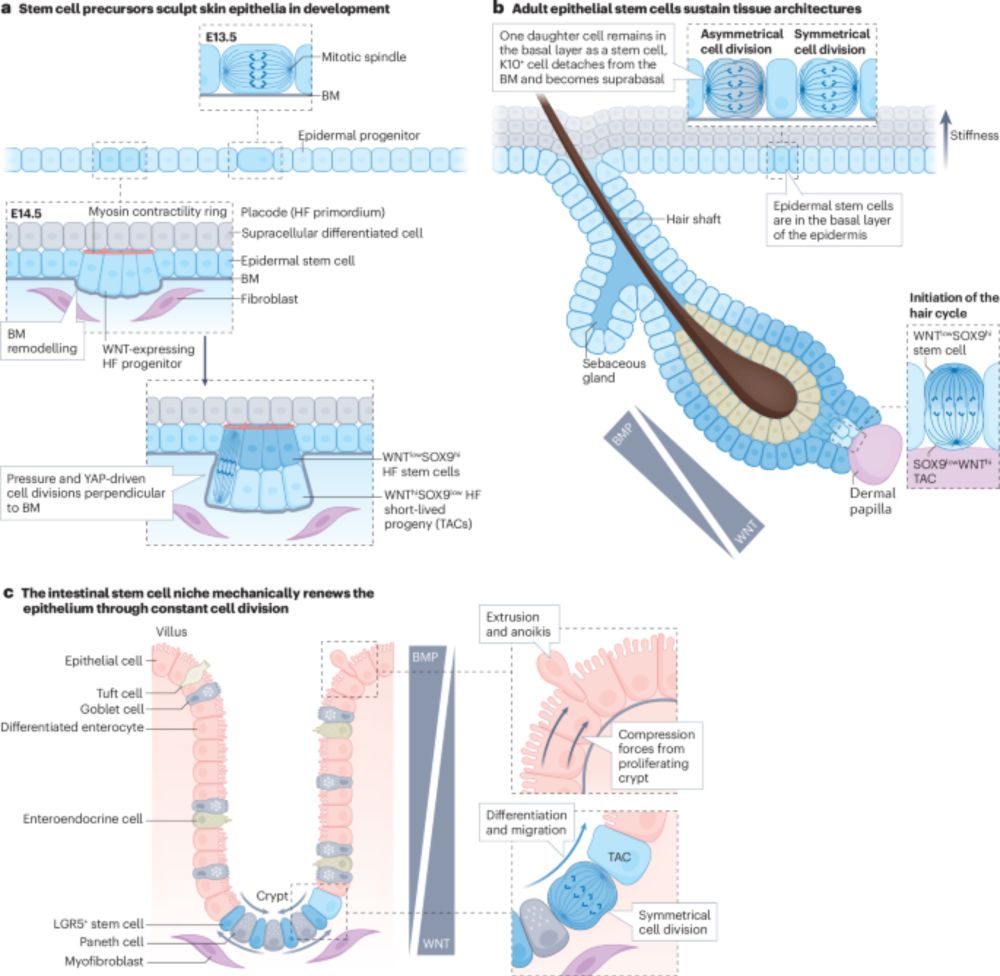
@jorgealmagro.bsky.social, @thefuchslab.bsky.social et al. discuss the role of stem cell mechanics during developmental processes and what happens when the mechanics of the stem cell niche are disrupted in cancer.
👉 go.nature.com/3EsJzJx
#StemCells #Mechanobiology #Cancer
🔍 Focus: AI-driven smart microscopy, machine learning, image analysis
📍 Location: Gothenburg, Sweden 🇸🇪
🗓Apply by: 24 Feb 2025
web103.reachmee.com/ext/I005/103...
🔍 Focus: AI-driven smart microscopy, machine learning, image analysis
📍 Location: Gothenburg, Sweden 🇸🇪
🗓Apply by: 24 Feb 2025
web103.reachmee.com/ext/I005/103...


