PhD @ UBC (Schafer)
BSc @ UVic (Rosenberg, Berg, and Leitch)
Interested in organometallic chemistry, catalysis, photochemistry, and organic methodology

chemsky 🧪
www.science.org/doi/10.1126/...

chemsky 🧪
www.science.org/doi/10.1126/...
www.youtube.com/watch?v=LZE9...

www.youtube.com/watch?v=LZE9...
www.nature.com/articles/s41...

www.nature.com/articles/s41...


pubs.acs.org/doi/10.1021/...
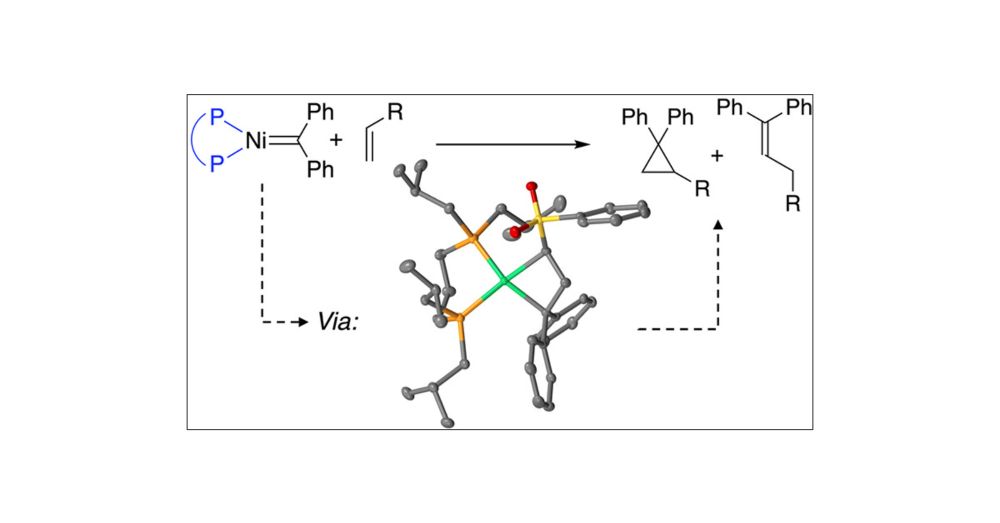
pubs.acs.org/doi/10.1021/...
Leitch lab folks (current and alumni) gave amazing talks, posters, workshops, and tore it up at the social events! Very proud of Kushal, Jae,
@gilianthomas.bsky.social, and Max & @camzheng.bsky.social (who couldn't make the photo)

Leitch lab folks (current and alumni) gave amazing talks, posters, workshops, and tore it up at the social events! Very proud of Kushal, Jae,
@gilianthomas.bsky.social, and Max & @camzheng.bsky.social (who couldn't make the photo)

#ChemSky #AcademicSky @pubs.acs.org
pubs.acs.org/doi/10.1021/...

#ChemSky #AcademicSky @pubs.acs.org
pubs.acs.org/doi/10.1021/...
👉 buff.ly/Rnbdtbm

👉 buff.ly/Rnbdtbm
Congrats to former member Cheng along with Thérèse, and Yulia!
Read more about it here:
pubs.acs.org/doi/10.1021/...
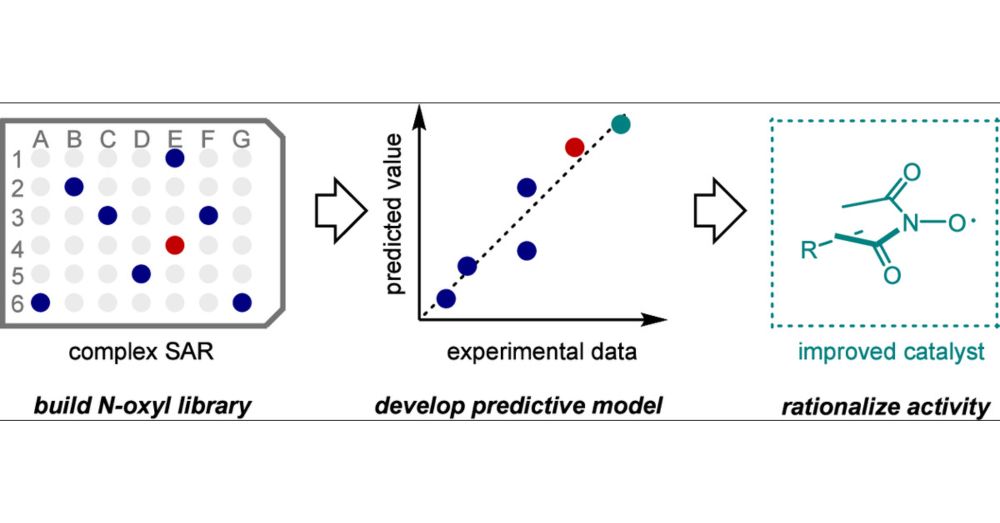
Congrats to former member Cheng along with Thérèse, and Yulia!
Read more about it here:
pubs.acs.org/doi/10.1021/...

Photos to come!
Photos to come!
Please repost and share! #chemsky #inorganicchemistry #PhD #PhDscholarship #catalysis #greenchem












