Anjana George
@anjanageorge.bsky.social
PhD student at IISER Bhopal | Biophysics | Membrane protein folding | Currently looking for a postdoc position
Reposted by Anjana George
The amazing Sofia Lövestam initiated the below project, when she became interested in the vault particles that we sometimes observe in #cryoEM images of brain-derived #amyloid filaments.
www.biorxiv.org/content/10.1...
www.biorxiv.org/content/10.1...

Cryo-EM structure of the vault from human brain reveals symmetry mismatch at its caps
The vault protein is expressed in most eukaryotic cells, where it is assembled on polyribosomes into large hollow barrel-shaped complexes. Despite its widespread and abundant presence in cells, the bi...
www.biorxiv.org
May 28, 2025 at 7:33 AM
The amazing Sofia Lövestam initiated the below project, when she became interested in the vault particles that we sometimes observe in #cryoEM images of brain-derived #amyloid filaments.
www.biorxiv.org/content/10.1...
www.biorxiv.org/content/10.1...
Reposted by Anjana George
Very special feelings to announce this one... A project that started like 10 years ago is reaching the finish line, ready to shine. In a dream-team with @beckmannlab.bsky.social we solved the long-chased structure of the active membrane protein insertase SecYEG-YidC
www.biorxiv.org/content/10.1...
www.biorxiv.org/content/10.1...

Substrate-induced assembly and functional mechanism of the bacterial membrane protein insertase SecYEG-YidC
The universally conserved Sec translocon and the YidC/Oxa1-type insertases mediate biogenesis of alpha-helical membrane proteins, but the molecular basis of their cooperation has remained disputed over decades. A recent discovery of a multi-subunit insertase in eukaryotes has raised the question about the architecture of the putative bacterial ortholog SecYEG-YidC and its functional mechanism. Here, we combine cryogenic electron microscopy with cell-free protein synthesis in nanodiscs to visualize biogenesis of the polytopic membrane protein NuoK, the subunit K of NADH-quinone oxidoreductase, that requires both SecYEG and YidC for insertion. We demonstrate that YidC is recruited to the back of the translocon at the late stage of the substrate insertion, in resemblance to the eukaryotic system, and in vivo experiments indicate that the complex assembly is vital for the cells. The nascent chain does not utilize the lateral gate of SecYEG, but enters the lipid membrane at the SecYE-YidC interface, with YidC being the primary insertase. SecYEG-YidC complex promotes folding of the nascent helices at the interface prior their insertion, so the examined cellular pathway follows the fundamental thermodynamic principles of membrane protein folding. Our data provide the first detailed insight on the elusive insertase machinery in the physiologically relevant environment, highlight the importance of the nascent chain for its assembly, and prove the evolutionary conservation of the gate-independent insertion route. ### Competing Interest Statement The authors have declared no competing interest. Deutsche Forschungsgemeinschaft, https://ror.org/018mejw64, Ke1879/3, 267205415 (CRC 1208) European Research Council, https://ror.org/0472cxd90, CRYOTRANSLATION
www.biorxiv.org
May 27, 2025 at 9:21 AM
Very special feelings to announce this one... A project that started like 10 years ago is reaching the finish line, ready to shine. In a dream-team with @beckmannlab.bsky.social we solved the long-chased structure of the active membrane protein insertase SecYEG-YidC
www.biorxiv.org/content/10.1...
www.biorxiv.org/content/10.1...
Reposted by Anjana George
This is just an excellent preprint by Rasmus Jensen and colleagues from Julia Mahamid's lab @embl.org - a tour de force of Cryogenic electron tomography to do in cell structural biology in which they discover a new complex and solve its structure and function! www.biorxiv.org/content/10.1...

In-cell discovery and characterization of a non-canonical bacterial protein translocation-folding complex
Cryo-electron tomography has emerged as powerful technology for in-cell structural biology, and in combination with breakthroughs in protein structure prediction, offers a unique opportunity for illum...
www.biorxiv.org
May 22, 2025 at 7:01 PM
This is just an excellent preprint by Rasmus Jensen and colleagues from Julia Mahamid's lab @embl.org - a tour de force of Cryogenic electron tomography to do in cell structural biology in which they discover a new complex and solve its structure and function! www.biorxiv.org/content/10.1...
Reposted by Anjana George
Researchers have discovered a new antibiotic molecule in soil samples from a laboratory technician's garden
https://go.nature.com/43qStll
https://go.nature.com/43qStll

New antibiotic that kills drug-resistant bacteria discovered in technician’s garden
The molecule targets bacteria in a way that other drugs don’t.
go.nature.com
March 26, 2025 at 5:10 PM
Researchers have discovered a new antibiotic molecule in soil samples from a laboratory technician's garden
https://go.nature.com/43qStll
https://go.nature.com/43qStll
Reposted by Anjana George
Out just now @naturecomms.bsky.social: using cryo-EM and cryo-electron tomography, we provide evidence that structurally heterogeneous ribosomes can cooperate in protein synthesis in bacterial cells:
www.nature.com/articles/s41...
www.nature.com/articles/s41...
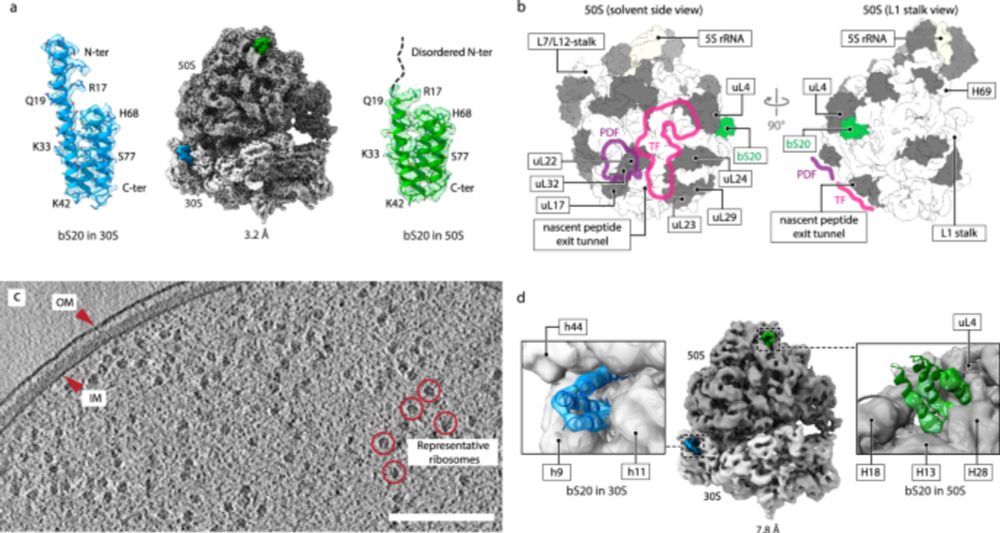
Structurally heterogeneous ribosomes cooperate in protein synthesis in bacterial cells - Nature Communications
Cells can simultaneously produce structurally dissimilar ribosomes, suggesting functional specialization of distinct ribosome populations. Here, the authors show that distinct ribosomes cooperate rath...
www.nature.com
March 21, 2025 at 8:50 AM
Out just now @naturecomms.bsky.social: using cryo-EM and cryo-electron tomography, we provide evidence that structurally heterogeneous ribosomes can cooperate in protein synthesis in bacterial cells:
www.nature.com/articles/s41...
www.nature.com/articles/s41...
Reposted by Anjana George
I'm super happy that our story is now published!
📖 www.science.org/doi/10.1126/...
But what changed compared to the original preprint?
Also, I feel i should post Movie 1 🎥, that inspired the cover. Back when I did the original bluesky thread, movies were not available.
📖 www.science.org/doi/10.1126/...
But what changed compared to the original preprint?
Also, I feel i should post Movie 1 🎥, that inspired the cover. Back when I did the original bluesky thread, movies were not available.
March 21, 2025 at 2:09 PM
I'm super happy that our story is now published!
📖 www.science.org/doi/10.1126/...
But what changed compared to the original preprint?
Also, I feel i should post Movie 1 🎥, that inspired the cover. Back when I did the original bluesky thread, movies were not available.
📖 www.science.org/doi/10.1126/...
But what changed compared to the original preprint?
Also, I feel i should post Movie 1 🎥, that inspired the cover. Back when I did the original bluesky thread, movies were not available.
Beautiful!
In a new Science study, cryo–electron tomography captures the in-cell architecture of the mitochondrial respiratory chain, illuminating how the coordinated action of molecular machines drives life’s fundamental energy conversion.
Learn more in this week's issue: scim.ag/3FA3Ygq
Learn more in this week's issue: scim.ag/3FA3Ygq
March 22, 2025 at 6:17 PM
Beautiful!
Reposted by Anjana George
In a new Science study, cryo–electron tomography captures the in-cell architecture of the mitochondrial respiratory chain, illuminating how the coordinated action of molecular machines drives life’s fundamental energy conversion.
Learn more in this week's issue: scim.ag/3FA3Ygq
Learn more in this week's issue: scim.ag/3FA3Ygq
March 20, 2025 at 6:05 PM
In a new Science study, cryo–electron tomography captures the in-cell architecture of the mitochondrial respiratory chain, illuminating how the coordinated action of molecular machines drives life’s fundamental energy conversion.
Learn more in this week's issue: scim.ag/3FA3Ygq
Learn more in this week's issue: scim.ag/3FA3Ygq

