
tinyurl.com/ChattScience
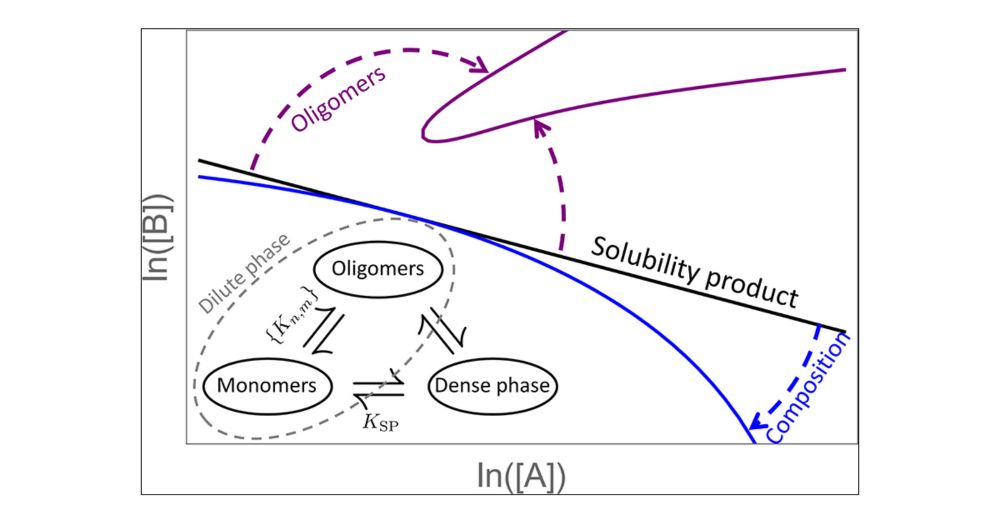



Like palm reading for your phase boundary.
Plus, when is your condensate actually an oligomer (or vice versa)?
A new preprint from the Schmit Group, in collaboration with Jonathon Ditlev, Les Loew, and @ani-chattaraj.bsky.social
www.biorxiv.org/content/10.1...

Like palm reading for your phase boundary.
Plus, when is your condensate actually an oligomer (or vice versa)?
A new preprint from the Schmit Group, in collaboration with Jonathon Ditlev, Les Loew, and @ani-chattaraj.bsky.social
www.biorxiv.org/content/10.1...
Here's a glimpse.
See you in the next session!
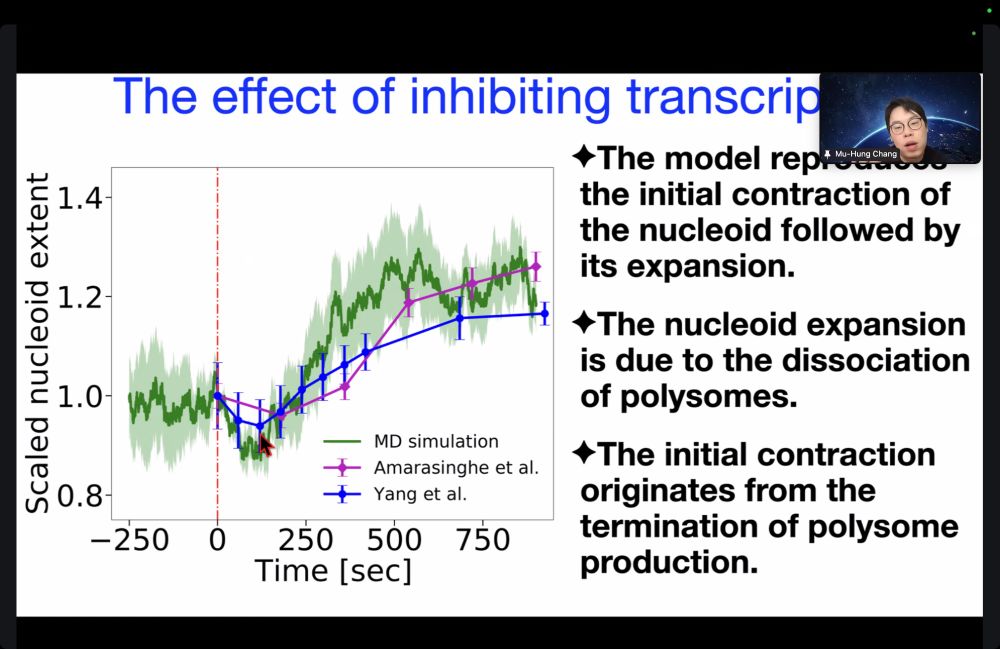
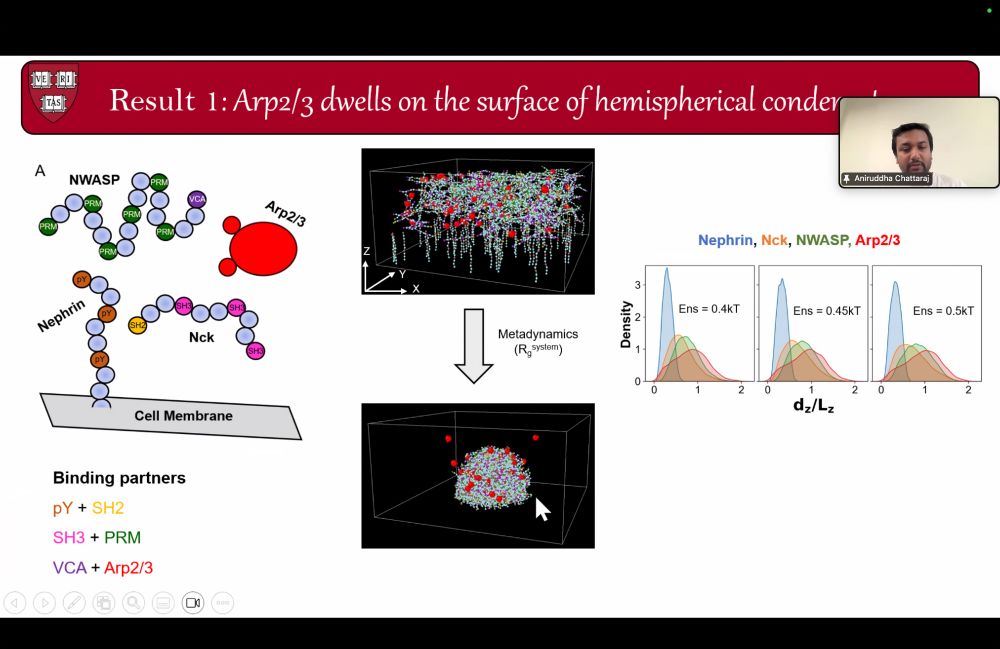
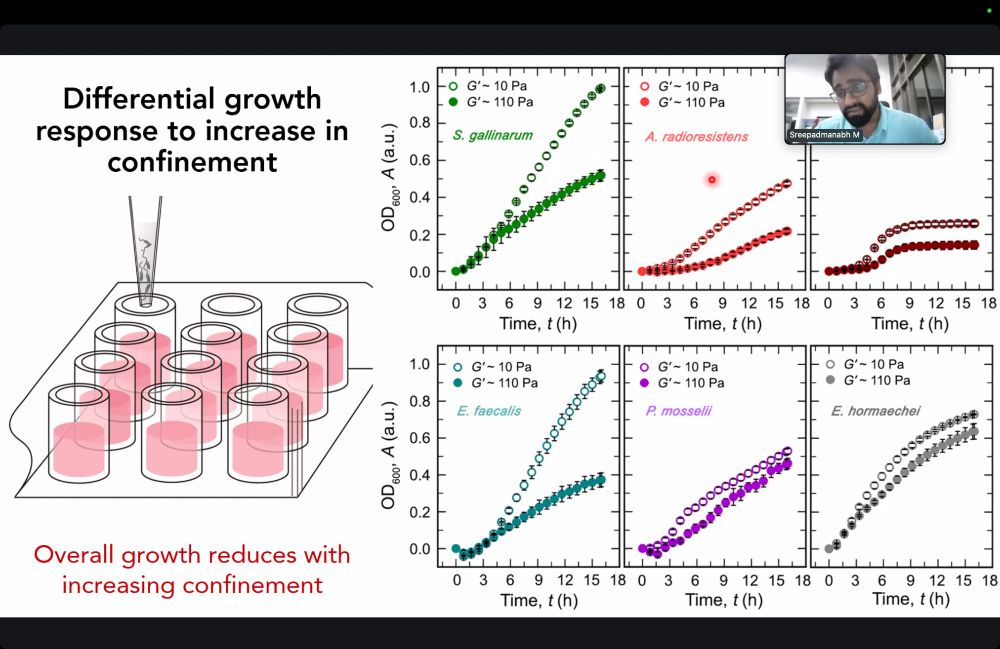
Here's a glimpse.
See you in the next session!

doi.org/10.1016/j.bp...
doi.org/10.1016/j.bp...

