
This work was a product of years of dedication and countless hours of research in the labs of Joanna Wysocka and Dan Jarosz labs @stanfordmedicine.bsky.social
www.cell.com/cell/fulltex...
Special thanks to the reviewers whose comments improved our manuscript a lot! rdcu.be/eI3tD

Special thanks to the reviewers whose comments improved our manuscript a lot! rdcu.be/eI3tD
med.stanford.edu/news/insight...

med.stanford.edu/news/insight...
connecting ‘cell surface RNA biology’ to cancer biology
@natbiotech.nature.com
www.nature.com/articles/s41...
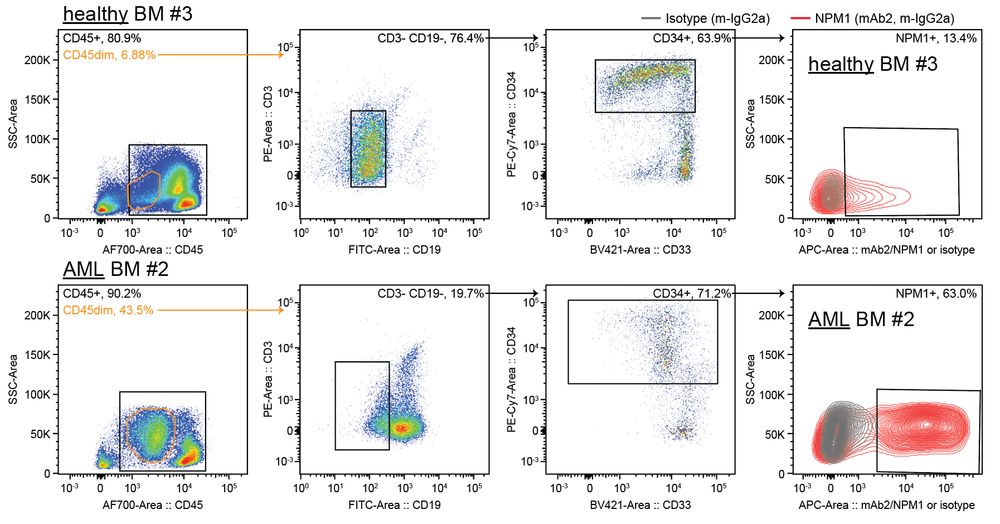
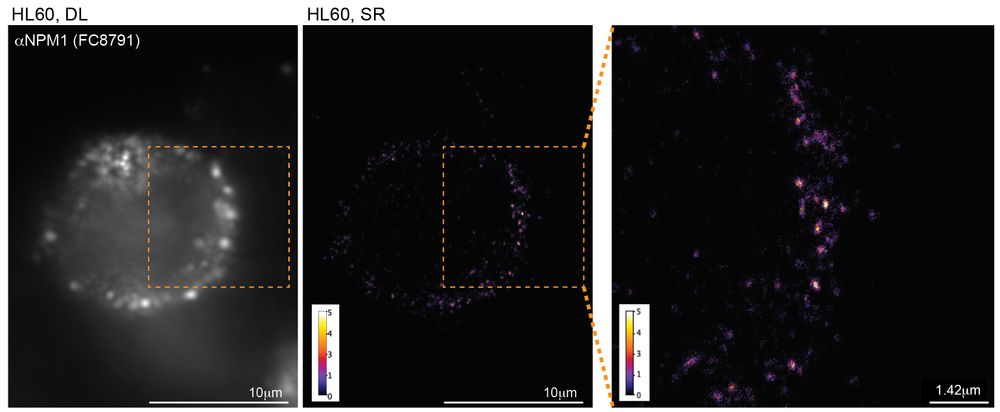
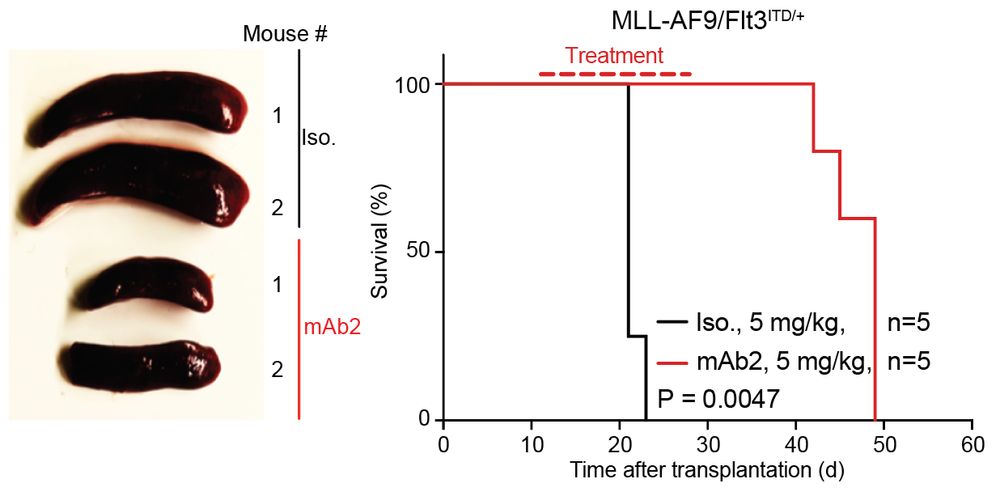
connecting ‘cell surface RNA biology’ to cancer biology
@natbiotech.nature.com
www.nature.com/articles/s41...
Fascinating new @cp-cell.bsky.social study by @shadys11.bsky.social D. Jarosz & J. Wysocka, uncovering a molecular function for these 2 substitutions in promoting the solubility of FOXP2. (1/n)

Fascinating new @cp-cell.bsky.social study by @shadys11.bsky.social D. Jarosz & J. Wysocka, uncovering a molecular function for these 2 substitutions in promoting the solubility of FOXP2. (1/n)

This work was a product of years of dedication and countless hours of research in the labs of Joanna Wysocka and Dan Jarosz labs @stanfordmedicine.bsky.social
www.cell.com/cell/fulltex...

This work was a product of years of dedication and countless hours of research in the labs of Joanna Wysocka and Dan Jarosz labs @stanfordmedicine.bsky.social
www.cell.com/cell/fulltex...


