
Google Scholar: https://scholar.google.co.uk/citations?user=HZ2wNeEAAAAJ&hl=en
ORCID: https://orcid.org/0000-0002-9311-6920

Are you planning, conducting, or reporting an early phase dose finding trial (with dose [de]escalation strategies? This CONSORT Extension will help you produce a transparent and adequately reported trial results report. Use it throughout the trial cycle!
www.sciencedirect.com/science/arti...
Read about life and work that made this statistician a "citation millionaire"
#BMJChristmas
www.bmj.com/content/391/...

Read about life and work that made this statistician a "citation millionaire"
#BMJChristmas
www.bmj.com/content/391/...
--> doi.org/10.1016/j.la...
#AI #Machinelearning #predictiveAI

--> doi.org/10.1016/j.la...
#AI #Machinelearning #predictiveAI
But these decreases are counterbalanced by increases in other regions
www.bmj.com/content/391/...

But these decreases are counterbalanced by increases in other regions
www.bmj.com/content/391/...
doi.org/10.1136/bmj....

doi.org/10.1136/bmj....
Now O2 has broken the taboo, the big risk is other firms will likely follow suit... the Govt must step in to stop mobile, broadband and pay-TV firms increasing prices mid-contract by MORE than they said they would when people sign up.



Now O2 has broken the taboo, the big risk is other firms will likely follow suit... the Govt must step in to stop mobile, broadband and pay-TV firms increasing prices mid-contract by MORE than they said they would when people sign up.

Kudos to France and Bayer for working on this.
www.nejm.org/doi/full/10....

Kudos to France and Bayer for working on this.
www.nejm.org/doi/full/10....
retractionwatch.com/2025/10/03/w...

retractionwatch.com/2025/10/03/w...
statmodeling.stat.columbia.edu/2025/10/09/t...
statmodeling.stat.columbia.edu/2025/10/09/t...
My USA colleagues are in a find-out phase!!
My USA colleagues are in a find-out phase!!
@amstatnews.bsky.social #openaccess www.tandfonline.com/doi/full/10....

@amstatnews.bsky.social #openaccess www.tandfonline.com/doi/full/10....
onlinelibrary.wiley.com/doi/10.1002/...

onlinelibrary.wiley.com/doi/10.1002/...
bmcmedicine.biomedcentral.com/articles/10....

bmcmedicine.biomedcentral.com/articles/10....
#EpiSky #CausalSky
jamanetwork.com/journals/jam...

#EpiSky #CausalSky
jamanetwork.com/journals/jam...
onlinelibrary.wiley.com/doi/10.1002/...

onlinelibrary.wiley.com/doi/10.1002/...


Killing mRNA vaccines will kill people.
open.substack.com/pub/rasmusse...

Killing mRNA vaccines will kill people.
open.substack.com/pub/rasmusse...
@mgtmccartney.bsky.social and @debscohen.bsky.social investigate
www.bmj.com/content/390/...

@mgtmccartney.bsky.social and @debscohen.bsky.social investigate
www.bmj.com/content/390/...
www.linkedin.com/posts/munyad...

www.linkedin.com/posts/munyad...
academic.oup.com/biometrics/a...
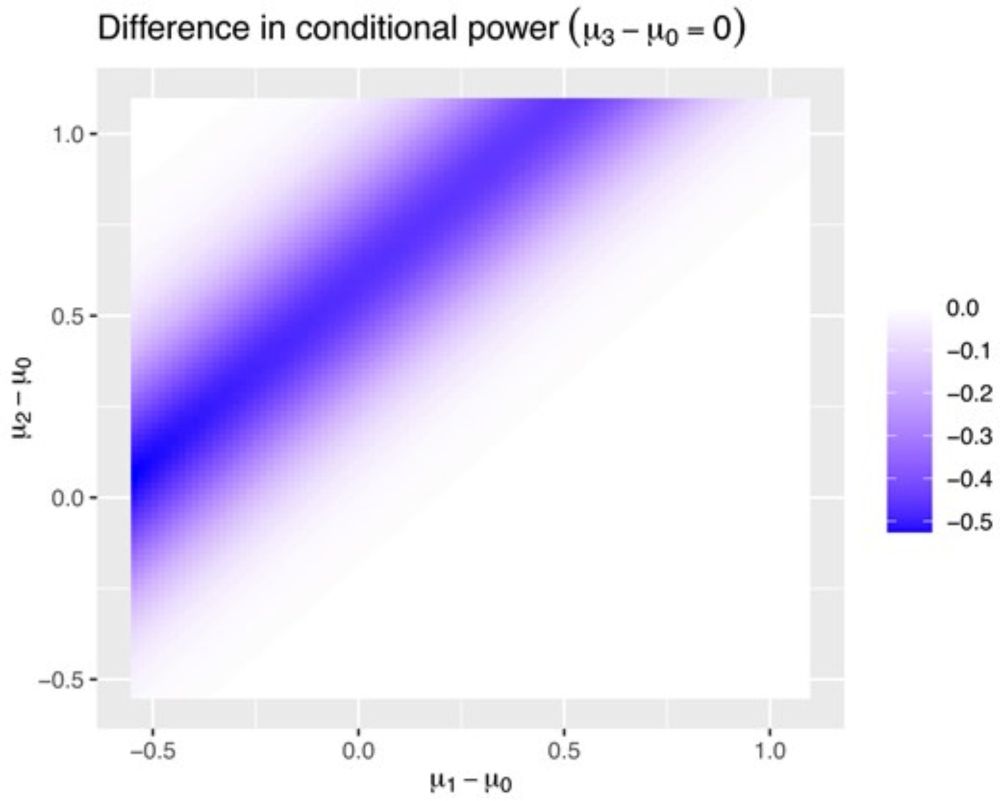
academic.oup.com/biometrics/a...


