Passionate about breast cancer care and research
👉🏽 Free CME 🔗 integrityce.com/TBT2024
Which is likely to be optimal for HR+ mBC that progresses after 1Y of 1L ribociclib + AI and harbors an ESR1 Y537S mutation?
Abemaciclib + fulvestrant
Imlunestrant + abemaciclib
Elacestrant
Capivasertib + fulvestrant

👉🏽 Free CME 🔗 integrityce.com/TBT2024
Which is likely to be optimal for HR+ mBC that progresses after 1Y of 1L ribociclib + AI and harbors an ESR1 Y537S mutation?
Abemaciclib + fulvestrant
Imlunestrant + abemaciclib
Elacestrant
Capivasertib + fulvestrant
👉🏽#CME Eval 🔗 integrityce.com/TBTeval24
👉🏽ALL CME🔗 integrityce.com/TBT2024
Per INAVO120 data in ET-refractory, PIK3CA+ HR+ la/mBC, adding inavolisib to palbociclib + fulvestrant improved mPFS to:
9 months
12 months
15 months
18 months

👉🏽#CME Eval 🔗 integrityce.com/TBTeval24
👉🏽ALL CME🔗 integrityce.com/TBT2024
Per INAVO120 data in ET-refractory, PIK3CA+ HR+ la/mBC, adding inavolisib to palbociclib + fulvestrant improved mPFS to:
9 months
12 months
15 months
18 months
🔑points
👉Novel ET are changing trt landscape for ER+/HER2- ABC
🤔W/ expanding options, come opportunities and challenges
➡️Many burning questions: Sequencing therapy, new combinations (ie CDK4/6i+SERDs)...

🔑points
👉Novel ET are changing trt landscape for ER+/HER2- ABC
🤔W/ expanding options, come opportunities and challenges
➡️Many burning questions: Sequencing therapy, new combinations (ie CDK4/6i+SERDs)...
👉For instance: Feb 2024, ARV-471, an investigational, oral PROTAC studied in the phase 3 VERITAC-2 trial, received FDA fast-track designation.
www.pfizer.com/news/announc...
👉For instance: Feb 2024, ARV-471, an investigational, oral PROTAC studied in the phase 3 VERITAC-2 trial, received FDA fast-track designation.
www.pfizer.com/news/announc...
Beyond SERDs/SERMs…novel endocrine therapies targeting other mechanisms in pipeline:
Proteolysis targeting chimeras (PROTACs), selective ER covalent antagonists, complete ER antagonists just to name a few!🤯

Beyond SERDs/SERMs…novel endocrine therapies targeting other mechanisms in pipeline:
Proteolysis targeting chimeras (PROTACs), selective ER covalent antagonists, complete ER antagonists just to name a few!🤯
Beyond EMBER-3, novel combinations with SERDs and SERMs also being evaluated with CDK4/6i

Beyond EMBER-3, novel combinations with SERDs and SERMs also being evaluated with CDK4/6i
➡️Multiple novel SERDS studied in HR+/HER2- ABC
👍Elacestrant currently remains the only FDA approved option
🚧Novel SERDs w/ unique AEs from fulvestrant
⚡This is a rapidly changing landscape!


➡️Multiple novel SERDS studied in HR+/HER2- ABC
👍Elacestrant currently remains the only FDA approved option
🚧Novel SERDs w/ unique AEs from fulvestrant
⚡This is a rapidly changing landscape!
🔙 to our case
EMBER3 would be a great option, but based on availability, pt got elacestrant
🚫 abema+fulvestrant:
-ESR1 Y537S 👉fulvestrant resistance.
-POSTMONARCH👉modest PFS benof abema+fulv vs placebo+fulv AND 🚫benefit in subgroup w/ prior ribo
🔙 to our case
EMBER3 would be a great option, but based on availability, pt got elacestrant
🚫 abema+fulvestrant:
-ESR1 Y537S 👉fulvestrant resistance.
-POSTMONARCH👉modest PFS benof abema+fulv vs placebo+fulv AND 🚫benefit in subgroup w/ prior ribo
👩⚕️Mini Tutorial 6👩⚕️
Imlunestrant: favorable safety profile vs SoC. Dc rate: 4%
Imlun+abema: diarrhea=most common AE, all grade: 86%, grade≥3: 8%. Dc rate: 6%
No 👁️or 💙AE signals like in other oral SERDS (i.e. photopsia, bradycardia in camizestrant)

👩⚕️Mini Tutorial 6👩⚕️
Imlunestrant: favorable safety profile vs SoC. Dc rate: 4%
Imlun+abema: diarrhea=most common AE, all grade: 86%, grade≥3: 8%. Dc rate: 6%
No 👁️or 💙AE signals like in other oral SERDS (i.e. photopsia, bradycardia in camizestrant)
👩⚕️Mini Tutorial 5👩⚕️
🔑Findings:
PFS ESR1m: 5.5 (Imlun) vs 3.8 mon (SoC). HR= 0.62, p<0.001
PFS all: 9.4 (Imlun+abema) vs 5.5 mon (imlun). HR=0.57, p<0.001
Benefit of imlun+abema in key subgroups: ESR1m, PIK3CAm, visceral disease, prior CDK4/6i



👩⚕️Mini Tutorial 5👩⚕️
🔑Findings:
PFS ESR1m: 5.5 (Imlun) vs 3.8 mon (SoC). HR= 0.62, p<0.001
PFS all: 9.4 (Imlun+abema) vs 5.5 mon (imlun). HR=0.57, p<0.001
Benefit of imlun+abema in key subgroups: ESR1m, PIK3CAm, visceral disease, prior CDK4/6i
👩⚕️Mini Tutorial 4👩⚕️
Novel, oral SERDs in development w/ ⬆️efficacy ⬇️toxicities
👉💡imlunestrant, studied in the EMBER-3 trial presented at #SABCS24
🔑Pt features:
~37% with ESR1m, ~40% with PIK3CAm, >50% visceral disease
👉1/3 adj ET, 2/3 prior CDK4/6i

👩⚕️Mini Tutorial 4👩⚕️
Novel, oral SERDs in development w/ ⬆️efficacy ⬇️toxicities
👉💡imlunestrant, studied in the EMBER-3 trial presented at #SABCS24
🔑Pt features:
~37% with ESR1m, ~40% with PIK3CAm, >50% visceral disease
👉1/3 adj ET, 2/3 prior CDK4/6i
2023, FDA approved elacestrant based on the EMERALD trial
Pts: prior 1-2L ET, ≤1 chemo for ABC: R 1:1 ➡️elacestrant vs SoC
➡️
PFS all: HR = 0.70; p = .002
PFS ESR1m: HR = 0.55; p = .0005
Benefit in key subgroups: PIK3CA mut, ESR1 mut, and TP53 mut
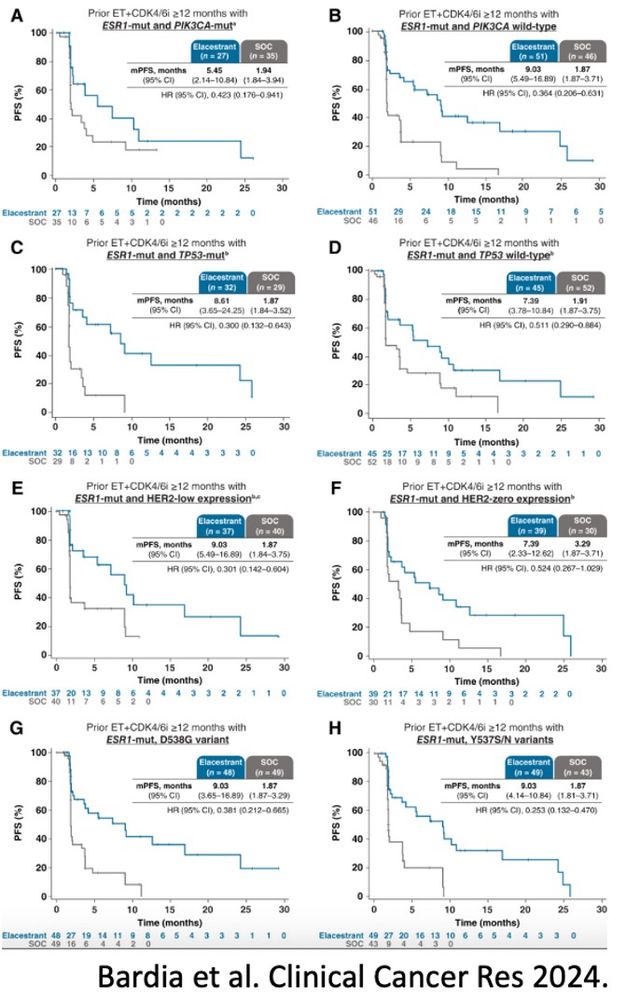
2023, FDA approved elacestrant based on the EMERALD trial
Pts: prior 1-2L ET, ≤1 chemo for ABC: R 1:1 ➡️elacestrant vs SoC
➡️
PFS all: HR = 0.70; p = .002
PFS ESR1m: HR = 0.55; p = .0005
Benefit in key subgroups: PIK3CA mut, ESR1 mut, and TP53 mut
👩⚕️Mini Tutorial 2👩⚕️
ESR1 mutations are present in:
~4% of 1L setting
~30% in 2L+ setting
👉Not all ESR1 mutations are created the same!
-ESR1 Y537S is associated with relative resistance to fulvestrant!
pmc.ncbi.nlm.nih.gov/articles/PMC...
👩⚕️Mini Tutorial 2👩⚕️
ESR1 mutations are present in:
~4% of 1L setting
~30% in 2L+ setting
👉Not all ESR1 mutations are created the same!
-ESR1 Y537S is associated with relative resistance to fulvestrant!
pmc.ncbi.nlm.nih.gov/articles/PMC...
👩⚕️Mini Tutorial 1👩⚕️
Therapy options after progression on 1L ET+CDK4/6i are biomarker driven.
@dr-rshatsky.bsky.social

👩⚕️Mini Tutorial 1👩⚕️
Therapy options after progression on 1L ET+CDK4/6i are biomarker driven.
@dr-rshatsky.bsky.social
What would you select as next line of therapy?
1. Abemaciclib+fulvestrant
2. Elacestrant
3. Evaluate for clinical trials involving novel SERDs
@dr-rshatsky.bsky.social
What would you select as next line of therapy?
1. Abemaciclib+fulvestrant
2. Elacestrant
3. Evaluate for clinical trials involving novel SERDs
@dr-rshatsky.bsky.social
⭐Case 2⭐
50 y/o postmenopausal F with de novo metastatic invasive ductal carcinoma, ER >95%, PR >95%, HER2– (IHC2+) involving the 🦴 and liver (intact function), progressed on 1st line ribociclib+AI after 12 months, w/ ESR1 Y537S mutation on ctDNA🤔
@dr-rshatsky.bsky.social
⭐Case 2⭐
50 y/o postmenopausal F with de novo metastatic invasive ductal carcinoma, ER >95%, PR >95%, HER2– (IHC2+) involving the 🦴 and liver (intact function), progressed on 1st line ribociclib+AI after 12 months, w/ ESR1 Y537S mutation on ctDNA🤔
@dr-rshatsky.bsky.social
⚖️ efficacy with safety…
Inavo arm: ↑AEs vs control
All-grade hyperglycemia: 58.6%; grade 3 or 4: 5.6%
All-grade stomatitis: 51.2%; grade 3 or 4: 5.6%
All-grade diarrhea: 48.1%; grade 3 or 4: 3.7%
1° ppx for above was not offered in the trial
@dr-rshatsky.bsky.social

⚖️ efficacy with safety…
Inavo arm: ↑AEs vs control
All-grade hyperglycemia: 58.6%; grade 3 or 4: 5.6%
All-grade stomatitis: 51.2%; grade 3 or 4: 5.6%
All-grade diarrhea: 48.1%; grade 3 or 4: 3.7%
1° ppx for above was not offered in the trial
@dr-rshatsky.bsky.social
Back to our case🔎
Pt started inavo, palbo, and fulv
2 weeks in, developed grade 2 stomatitis self-dced inavo and palbo
Started dexamethasone+mucositis mouthwash, but sxs not resolved
Currently undergoing evaluation for subsequent therapy
@dr-rshatsky.bsky.social

Back to our case🔎
Pt started inavo, palbo, and fulv
2 weeks in, developed grade 2 stomatitis self-dced inavo and palbo
Started dexamethasone+mucositis mouthwash, but sxs not resolved
Currently undergoing evaluation for subsequent therapy
@dr-rshatsky.bsky.social
👩⚕️Mini Tutorial 3👩⚕️
Pts R 1:1 to Inavo vs placebo +palbo+fulv
🔑findings:
Median f/u: 21.3 mon
Median PFS: 15.0 mon(Inavo) vs 7.3 mon (control) (HR 0.43; p<0.0001)
ORR: 58.4% (Inavo) vs 25% (control)
Consistent benefit across key subgroups
@dr-rshatsky.bsky.social

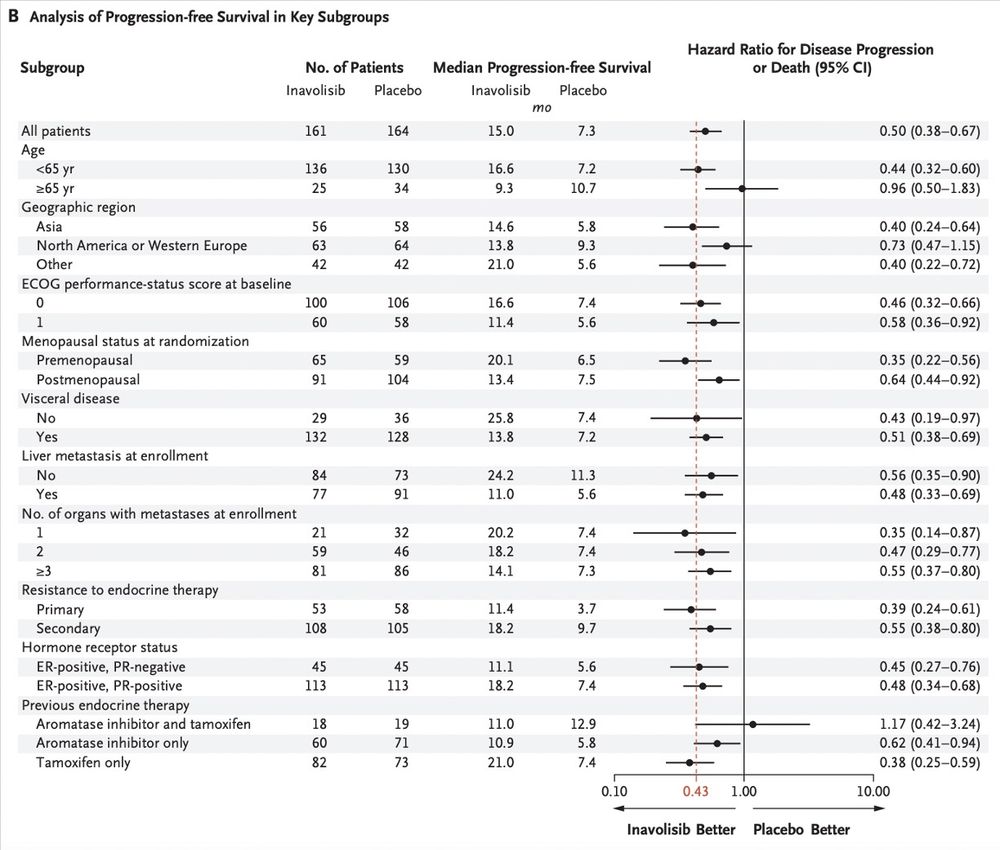
👩⚕️Mini Tutorial 3👩⚕️
Pts R 1:1 to Inavo vs placebo +palbo+fulv
🔑findings:
Median f/u: 21.3 mon
Median PFS: 15.0 mon(Inavo) vs 7.3 mon (control) (HR 0.43; p<0.0001)
ORR: 58.4% (Inavo) vs 25% (control)
Consistent benefit across key subgroups
@dr-rshatsky.bsky.social
👩⚕️Mini Tutorial 2👩⚕️
Pt characteristics in INAVO120:
No prior treatment for ABC
PIK3CA mut
a1c≤6.0, fasting BG<126
~Half with ≥3 involved organ sites
~Half with liver involvement, ~40% with 🫁 involvement
~1/3 with 1° endocrine resistance, 2/3 with 2°
@dr-rshatsky.bsky.social
👩⚕️Mini Tutorial 2👩⚕️
Pt characteristics in INAVO120:
No prior treatment for ABC
PIK3CA mut
a1c≤6.0, fasting BG<126
~Half with ≥3 involved organ sites
~Half with liver involvement, ~40% with 🫁 involvement
~1/3 with 1° endocrine resistance, 2/3 with 2°
@dr-rshatsky.bsky.social
👩⚕️Mini Tutorial 1👩⚕️
Oct 2024, FDA approved inavolisib, palbociclib, and fulvestrant in endocrine resistant HR+/HER2- advanced breast cancer, based on the phase 3, INAVO120 study
@dr-rshatsky.bsky.social

👩⚕️Mini Tutorial 1👩⚕️
Oct 2024, FDA approved inavolisib, palbociclib, and fulvestrant in endocrine resistant HR+/HER2- advanced breast cancer, based on the phase 3, INAVO120 study
@dr-rshatsky.bsky.social
Tissue NGS was not performed. CtDNA 🧬analysis revealing the following:
-PIK3CA H1047R
How does this impact your answer to the prior poll? 🤔
@dr-rshatsky.bsky.social
Tissue NGS was not performed. CtDNA 🧬analysis revealing the following:
-PIK3CA H1047R
How does this impact your answer to the prior poll? 🤔
@dr-rshatsky.bsky.social
Tissue NGS was not performed. CtDNA 🧬analysis revealing the following:
-PIK3CA H1047R
How does this impact your answer to the prior poll? 🤔
@dr-rshatsky.bsky.social
Tissue NGS was not performed. CtDNA 🧬analysis revealing the following:
-PIK3CA H1047R
How does this impact your answer to the prior poll? 🤔
@dr-rshatsky.bsky.social

