Passionate about breast cancer care and research

Beyond SERDs/SERMs…novel endocrine therapies targeting other mechanisms in pipeline:
Proteolysis targeting chimeras (PROTACs), selective ER covalent antagonists, complete ER antagonists just to name a few!🤯

Beyond SERDs/SERMs…novel endocrine therapies targeting other mechanisms in pipeline:
Proteolysis targeting chimeras (PROTACs), selective ER covalent antagonists, complete ER antagonists just to name a few!🤯
Beyond EMBER-3, novel combinations with SERDs and SERMs also being evaluated with CDK4/6i

Beyond EMBER-3, novel combinations with SERDs and SERMs also being evaluated with CDK4/6i
➡️Multiple novel SERDS studied in HR+/HER2- ABC
👍Elacestrant currently remains the only FDA approved option
🚧Novel SERDs w/ unique AEs from fulvestrant
⚡This is a rapidly changing landscape!


➡️Multiple novel SERDS studied in HR+/HER2- ABC
👍Elacestrant currently remains the only FDA approved option
🚧Novel SERDs w/ unique AEs from fulvestrant
⚡This is a rapidly changing landscape!
👩⚕️Mini Tutorial 6👩⚕️
Imlunestrant: favorable safety profile vs SoC. Dc rate: 4%
Imlun+abema: diarrhea=most common AE, all grade: 86%, grade≥3: 8%. Dc rate: 6%
No 👁️or 💙AE signals like in other oral SERDS (i.e. photopsia, bradycardia in camizestrant)

👩⚕️Mini Tutorial 6👩⚕️
Imlunestrant: favorable safety profile vs SoC. Dc rate: 4%
Imlun+abema: diarrhea=most common AE, all grade: 86%, grade≥3: 8%. Dc rate: 6%
No 👁️or 💙AE signals like in other oral SERDS (i.e. photopsia, bradycardia in camizestrant)
👩⚕️Mini Tutorial 5👩⚕️
🔑Findings:
PFS ESR1m: 5.5 (Imlun) vs 3.8 mon (SoC). HR= 0.62, p<0.001
PFS all: 9.4 (Imlun+abema) vs 5.5 mon (imlun). HR=0.57, p<0.001
Benefit of imlun+abema in key subgroups: ESR1m, PIK3CAm, visceral disease, prior CDK4/6i



👩⚕️Mini Tutorial 5👩⚕️
🔑Findings:
PFS ESR1m: 5.5 (Imlun) vs 3.8 mon (SoC). HR= 0.62, p<0.001
PFS all: 9.4 (Imlun+abema) vs 5.5 mon (imlun). HR=0.57, p<0.001
Benefit of imlun+abema in key subgroups: ESR1m, PIK3CAm, visceral disease, prior CDK4/6i
👩⚕️Mini Tutorial 4👩⚕️
Novel, oral SERDs in development w/ ⬆️efficacy ⬇️toxicities
👉💡imlunestrant, studied in the EMBER-3 trial presented at #SABCS24
🔑Pt features:
~37% with ESR1m, ~40% with PIK3CAm, >50% visceral disease
👉1/3 adj ET, 2/3 prior CDK4/6i

👩⚕️Mini Tutorial 4👩⚕️
Novel, oral SERDs in development w/ ⬆️efficacy ⬇️toxicities
👉💡imlunestrant, studied in the EMBER-3 trial presented at #SABCS24
🔑Pt features:
~37% with ESR1m, ~40% with PIK3CAm, >50% visceral disease
👉1/3 adj ET, 2/3 prior CDK4/6i
2023, FDA approved elacestrant based on the EMERALD trial
Pts: prior 1-2L ET, ≤1 chemo for ABC: R 1:1 ➡️elacestrant vs SoC
➡️
PFS all: HR = 0.70; p = .002
PFS ESR1m: HR = 0.55; p = .0005
Benefit in key subgroups: PIK3CA mut, ESR1 mut, and TP53 mut
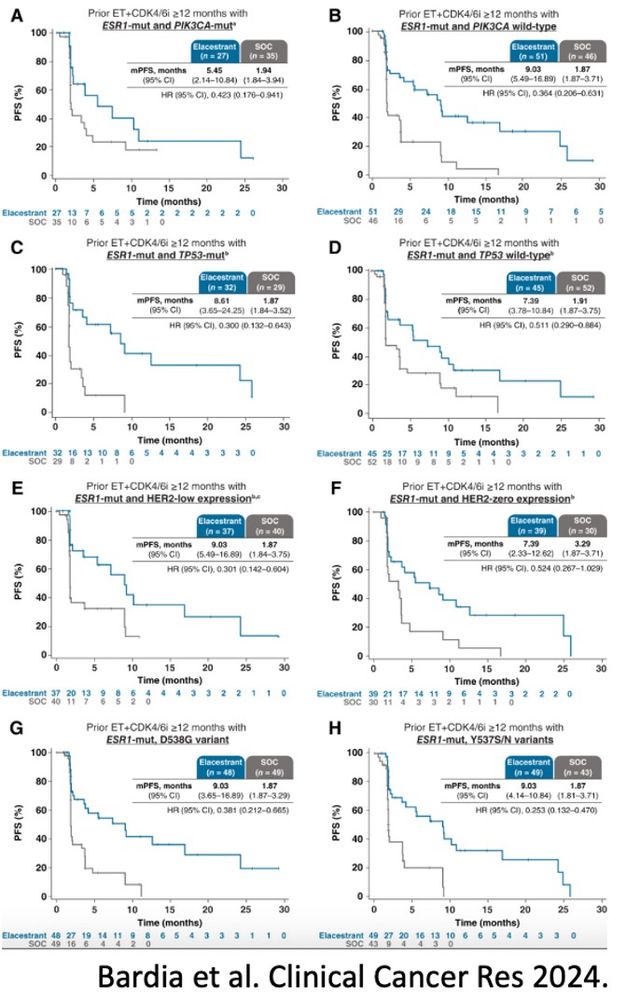
2023, FDA approved elacestrant based on the EMERALD trial
Pts: prior 1-2L ET, ≤1 chemo for ABC: R 1:1 ➡️elacestrant vs SoC
➡️
PFS all: HR = 0.70; p = .002
PFS ESR1m: HR = 0.55; p = .0005
Benefit in key subgroups: PIK3CA mut, ESR1 mut, and TP53 mut
👩⚕️Mini Tutorial 1👩⚕️
Therapy options after progression on 1L ET+CDK4/6i are biomarker driven.
@dr-rshatsky.bsky.social

👩⚕️Mini Tutorial 1👩⚕️
Therapy options after progression on 1L ET+CDK4/6i are biomarker driven.
@dr-rshatsky.bsky.social
⚖️ efficacy with safety…
Inavo arm: ↑AEs vs control
All-grade hyperglycemia: 58.6%; grade 3 or 4: 5.6%
All-grade stomatitis: 51.2%; grade 3 or 4: 5.6%
All-grade diarrhea: 48.1%; grade 3 or 4: 3.7%
1° ppx for above was not offered in the trial
@dr-rshatsky.bsky.social

⚖️ efficacy with safety…
Inavo arm: ↑AEs vs control
All-grade hyperglycemia: 58.6%; grade 3 or 4: 5.6%
All-grade stomatitis: 51.2%; grade 3 or 4: 5.6%
All-grade diarrhea: 48.1%; grade 3 or 4: 3.7%
1° ppx for above was not offered in the trial
@dr-rshatsky.bsky.social
👩⚕️Mini Tutorial 3👩⚕️
Pts R 1:1 to Inavo vs placebo +palbo+fulv
🔑findings:
Median f/u: 21.3 mon
Median PFS: 15.0 mon(Inavo) vs 7.3 mon (control) (HR 0.43; p<0.0001)
ORR: 58.4% (Inavo) vs 25% (control)
Consistent benefit across key subgroups
@dr-rshatsky.bsky.social

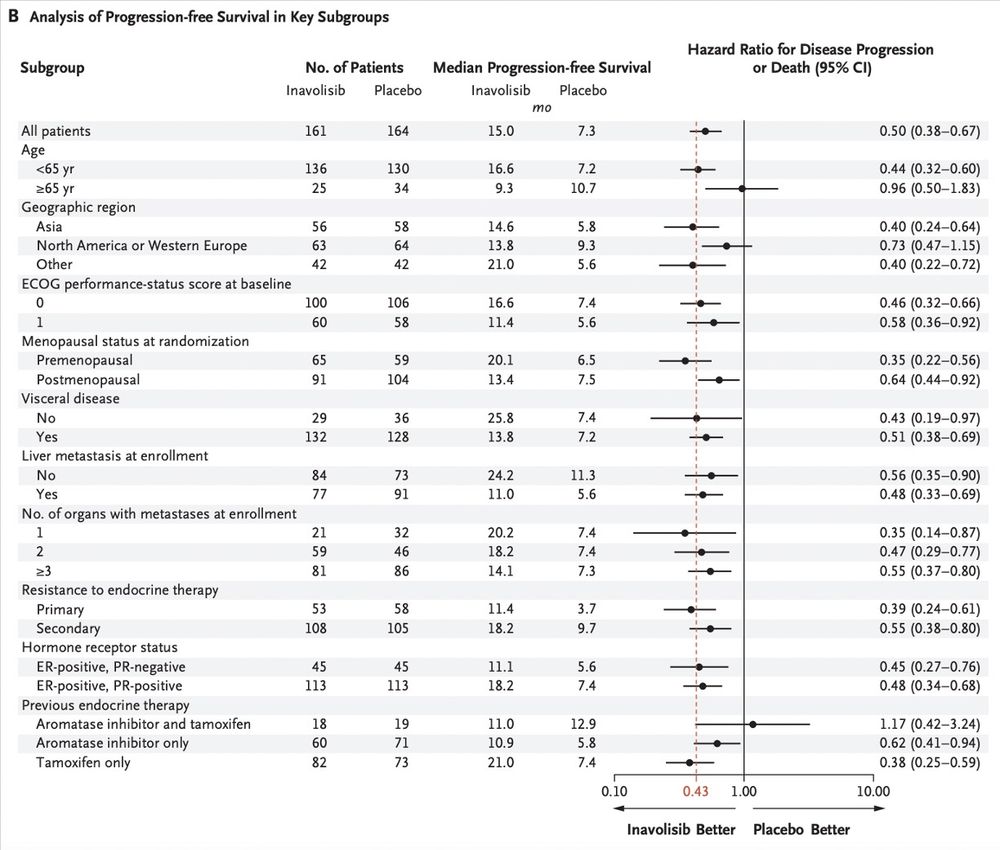
👩⚕️Mini Tutorial 3👩⚕️
Pts R 1:1 to Inavo vs placebo +palbo+fulv
🔑findings:
Median f/u: 21.3 mon
Median PFS: 15.0 mon(Inavo) vs 7.3 mon (control) (HR 0.43; p<0.0001)
ORR: 58.4% (Inavo) vs 25% (control)
Consistent benefit across key subgroups
@dr-rshatsky.bsky.social
👩⚕️Mini Tutorial 1👩⚕️
Oct 2024, FDA approved inavolisib, palbociclib, and fulvestrant in endocrine resistant HR+/HER2- advanced breast cancer, based on the phase 3, INAVO120 study
@dr-rshatsky.bsky.social

👩⚕️Mini Tutorial 1👩⚕️
Oct 2024, FDA approved inavolisib, palbociclib, and fulvestrant in endocrine resistant HR+/HER2- advanced breast cancer, based on the phase 3, INAVO120 study
@dr-rshatsky.bsky.social

