Tong Wang
@twangmdphd.bsky.social
Resident physician at Stanford Pathology | Greenleaf Lab | Penn MSTP | Interested in chemical biology, epigenetics, and clinically useful tests.
Reposted by Tong Wang
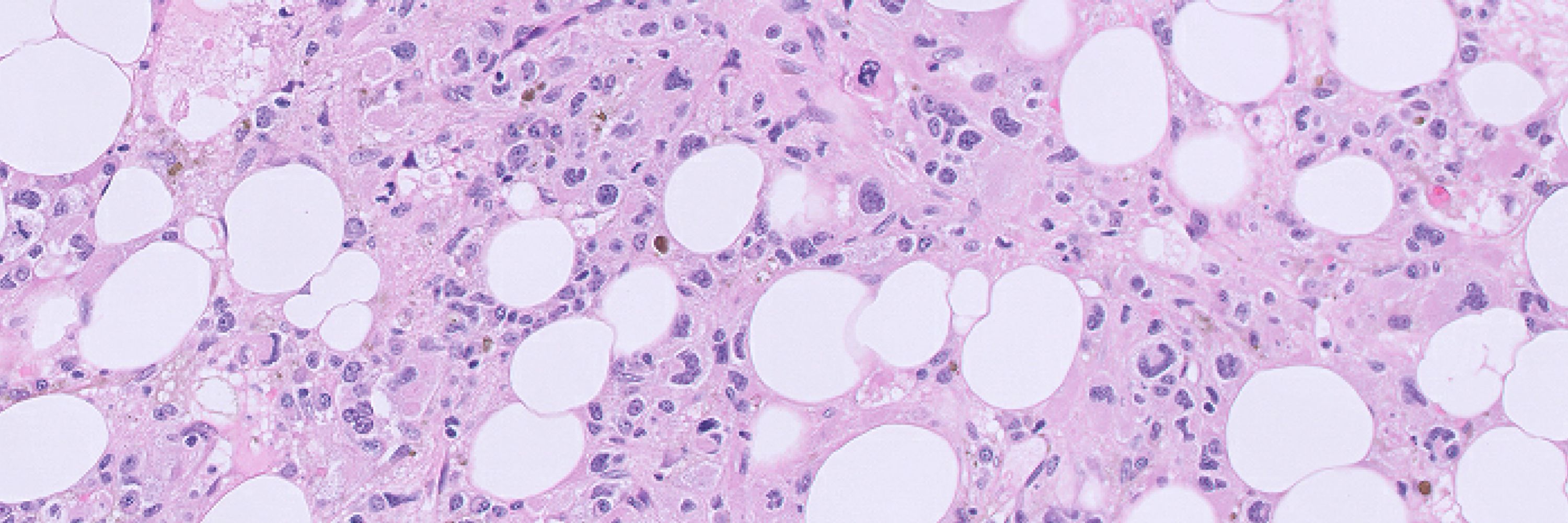
We then used sequence-to-activity deep learning models, to predict effects of non-coding edits on TF binding and chromatin accessibility. We first show that a ChromBPNet model can predict the same GATA site disruption mechanism exploited by the FDA-approved Casgevy medicine, specifically in T cells:

November 7, 2025 at 6:38 PM
We then used sequence-to-activity deep learning models, to predict effects of non-coding edits on TF binding and chromatin accessibility. We first show that a ChromBPNet model can predict the same GATA site disruption mechanism exploited by the FDA-approved Casgevy medicine, specifically in T cells:
8/ This was a group effort drawing expertise from different parts of the Greenleaf Lab and the larger Stanford Genetics community. A big thank you to @selinjessa.bsky.social, Georgi, Sandy, and @anshulkundaje.bsky.social I am especially grateful to Will for encouraging me to take on this project.
September 29, 2025 at 6:28 PM
8/ This was a group effort drawing expertise from different parts of the Greenleaf Lab and the larger Stanford Genetics community. A big thank you to @selinjessa.bsky.social, Georgi, Sandy, and @anshulkundaje.bsky.social I am especially grateful to Will for encouraging me to take on this project.
7/ There’s much more in the preprint (deep learning of novel T-cell non-coding off-targets, SpRY editors, and comparisons to other methods to name a few), but I won’t give it all away here. Base editors hold immense promise, and we hope our insights help to improve them.
September 29, 2025 at 6:28 PM
7/ There’s much more in the preprint (deep learning of novel T-cell non-coding off-targets, SpRY editors, and comparisons to other methods to name a few), but I won’t give it all away here. Base editors hold immense promise, and we hope our insights help to improve them.
6/ We also show how deep learning can predict the effects of non-coding edits. Here, an erythroid but not T-cell model predicts loss of DNA accessibility upon editing. The model learns the GATA motif driving this prediction. This site is of course the intended target of Casgevy.

September 29, 2025 at 6:28 PM
6/ We also show how deep learning can predict the effects of non-coding edits. Here, an erythroid but not T-cell model predicts loss of DNA accessibility upon editing. The model learns the GATA motif driving this prediction. This site is of course the intended target of Casgevy.
5/ We extensively optimized beCasKAS to be compatible with primary human T cells and 5moU mRNA delivery. Transient mRNA delivery limits off-target formation, which can be further mitigated by carefully selecting mRNA dose.

September 29, 2025 at 6:28 PM
5/ We extensively optimized beCasKAS to be compatible with primary human T cells and 5moU mRNA delivery. Transient mRNA delivery limits off-target formation, which can be further mitigated by carefully selecting mRNA dose.
4/ We are able to see the known strand-specific editing patterns of two contrasting editors: eBE (using hAPOBEC3A) and ABE8e. Using plasmid delivery in HEK293Ts, we find these two editors have surprisingly similar absolute editing frequencies.

September 29, 2025 at 6:28 PM
4/ We are able to see the known strand-specific editing patterns of two contrasting editors: eBE (using hAPOBEC3A) and ABE8e. Using plasmid delivery in HEK293Ts, we find these two editors have surprisingly similar absolute editing frequencies.
3/ beCasKAS makes two measurements at each off-target site. The unwound R-loop appears as a peak, and individual edits appear as mismatched nucleotides.

September 29, 2025 at 6:28 PM
3/ beCasKAS makes two measurements at each off-target site. The unwound R-loop appears as a peak, and individual edits appear as mismatched nucleotides.
2/ We use an N3-kethoxal pulldown probe because it has the same selectivity for ssDNA as the deaminases used in base editing. The cell permeable kethoxal allows us to enrich for unwound Cas9 R-loops that must occur before DNA editing. This selectivity underlies our sensitivity.

September 29, 2025 at 6:28 PM
2/ We use an N3-kethoxal pulldown probe because it has the same selectivity for ssDNA as the deaminases used in base editing. The cell permeable kethoxal allows us to enrich for unwound Cas9 R-loops that must occur before DNA editing. This selectivity underlies our sensitivity.

