
🎓 PhD cum laude in vaccinology, University of Amsterdam

1. A germline-targeting trimer can kickstart broadly neutralizing antibody lineages that target multiple epitopes succesfully.
1. A germline-targeting trimer can kickstart broadly neutralizing antibody lineages that target multiple epitopes succesfully.
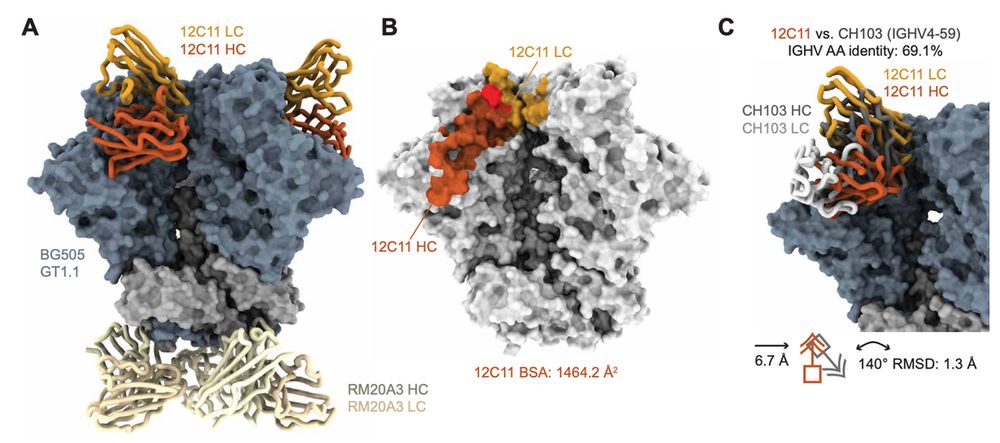



In non-human primates, we confirmed that there is a robust serum response to the epitope that we intended to generate antibodies against - the CD4 binding site.

In non-human primates, we confirmed that there is a robust serum response to the epitope that we intended to generate antibodies against - the CD4 binding site.


