Tony Cesare
@thecesarelab.bsky.social
Head of the Genome Integrity Unit, Children's Medical Research Institute. Professor, University of Sydney.
Thank you @karimmekhail.bsky.social, @chiololab.bsky.social, and @altmeyerlab.bsky.social for organising @fusionconf.bsky.social #SGO25.
Exciting science at a fantastic venue!
Exciting science at a fantastic venue!



November 3, 2025 at 11:53 AM
Thank you @karimmekhail.bsky.social, @chiololab.bsky.social, and @altmeyerlab.bsky.social for organising @fusionconf.bsky.social #SGO25.
Exciting science at a fantastic venue!
Exciting science at a fantastic venue!
Delighted to be in beautiful Riviera Maya Mexico 🇲🇽 for @fusionconf.bsky.social #SGO25.
www.fusion-conferences.com/conference/188
www.fusion-conferences.com/conference/188

October 31, 2025 at 4:10 PM
Delighted to be in beautiful Riviera Maya Mexico 🇲🇽 for @fusionconf.bsky.social #SGO25.
www.fusion-conferences.com/conference/188
www.fusion-conferences.com/conference/188
The Cesare Lab had an excellent time at the Australian Cell Cycle, DNA Repair & Telomere Meeting in Melbourne.
australiancellcycle.org
A huge thank you to @genomestability.bsky.social and all the organisers for putting together such a fantastic and inspiring meeting.
We're looking forward to 2027!
australiancellcycle.org
A huge thank you to @genomestability.bsky.social and all the organisers for putting together such a fantastic and inspiring meeting.
We're looking forward to 2027!

October 22, 2025 at 11:55 PM
The Cesare Lab had an excellent time at the Australian Cell Cycle, DNA Repair & Telomere Meeting in Melbourne.
australiancellcycle.org
A huge thank you to @genomestability.bsky.social and all the organisers for putting together such a fantastic and inspiring meeting.
We're looking forward to 2027!
australiancellcycle.org
A huge thank you to @genomestability.bsky.social and all the organisers for putting together such a fantastic and inspiring meeting.
We're looking forward to 2027!
My and @telomerase-cmri.bsky.social’s annual attempt to bring a little bit of college baseketball culture down under.
Go Tar Heels!
Go Tar Heels!
March 20, 2025 at 10:17 PM
My and @telomerase-cmri.bsky.social’s annual attempt to bring a little bit of college baseketball culture down under.
Go Tar Heels!
Go Tar Heels!
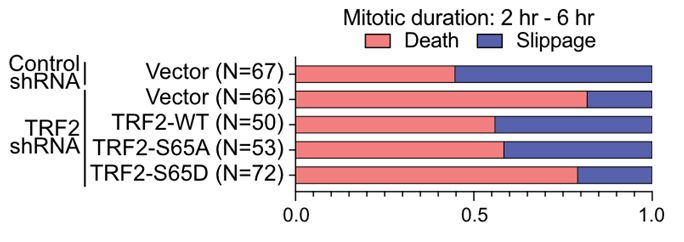
23/Live imaging from both labs revealed that TRF2 mutants that suppress MAD-telomere deprotection also reduce the prevalence of mitotic arrest driven lethality. While TRF2 mutants that promote MAD-telomere deprotection enhanced mitotic death or expedited time of mitotic arrest to lethality.
March 17, 2025 at 10:32 AM
23/Live imaging from both labs revealed that TRF2 mutants that suppress MAD-telomere deprotection also reduce the prevalence of mitotic arrest driven lethality. While TRF2 mutants that promote MAD-telomere deprotection enhanced mitotic death or expedited time of mitotic arrest to lethality.
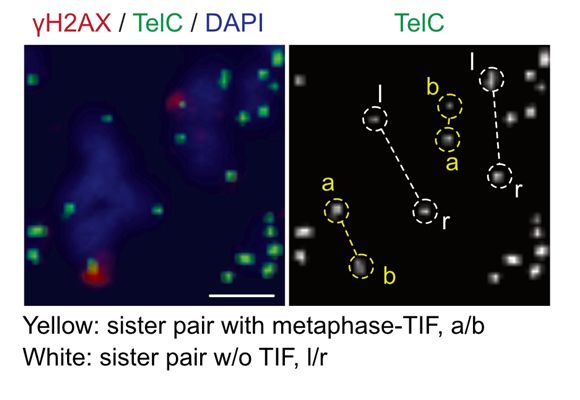
22/Of note, telomeres do not rapidly truncate in MAD-telomere deprotection like they do when the TRF2 basic domain is deleted. Thus, while the TRF2 basic domain is modified to enable BTR-dependent t-loop opening, it does not allow t-loop junction cleavage, consistent with a highly regulated pathway.
March 17, 2025 at 10:32 AM
22/Of note, telomeres do not rapidly truncate in MAD-telomere deprotection like they do when the TRF2 basic domain is deleted. Thus, while the TRF2 basic domain is modified to enable BTR-dependent t-loop opening, it does not allow t-loop junction cleavage, consistent with a highly regulated pathway.
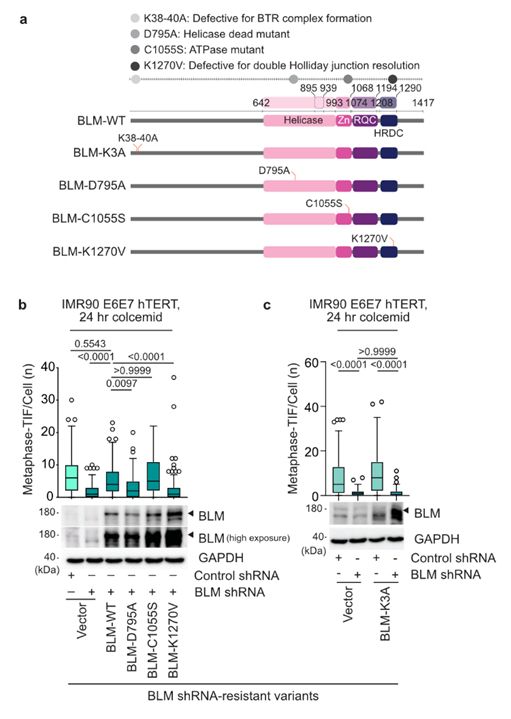
21/Diana’s molecular biology was consistent with the BTR complex being requisite for MAD-telomere deprotection following TRF2 modification.
This suggested that t-loops junctions are in a double Holliday Junction configuration during mitotic arrest before resolution by BTR.
This suggested that t-loops junctions are in a double Holliday Junction configuration during mitotic arrest before resolution by BTR.
March 17, 2025 at 10:32 AM
21/Diana’s molecular biology was consistent with the BTR complex being requisite for MAD-telomere deprotection following TRF2 modification.
This suggested that t-loops junctions are in a double Holliday Junction configuration during mitotic arrest before resolution by BTR.
This suggested that t-loops junctions are in a double Holliday Junction configuration during mitotic arrest before resolution by BTR.
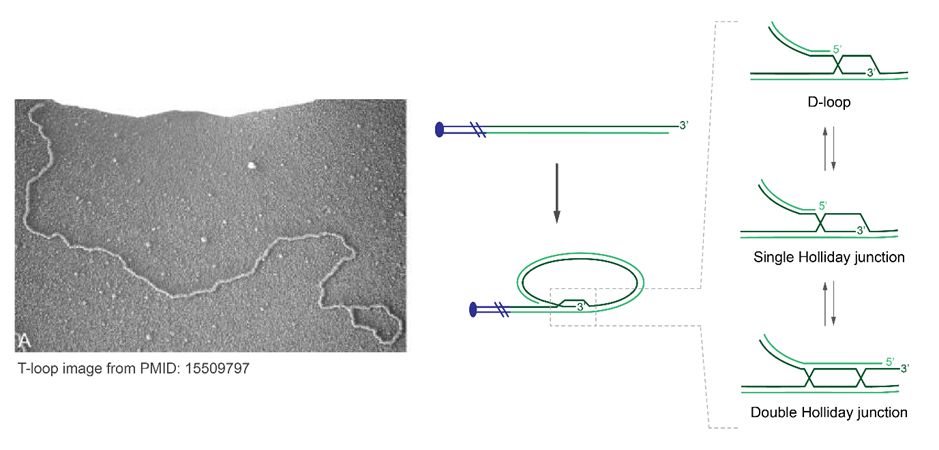
20/How does BLM fit into the story?
BLM does many things, including double Holliday Junction dissolution (dHJ) via the BTR complex. T-loops are typically drawn with strand-invasion promoting a displacement loop. However, this is conjecture, and the t-loop junction identify is unknown.
BLM does many things, including double Holliday Junction dissolution (dHJ) via the BTR complex. T-loops are typically drawn with strand-invasion promoting a displacement loop. However, this is conjecture, and the t-loop junction identify is unknown.
March 17, 2025 at 10:32 AM
20/How does BLM fit into the story?
BLM does many things, including double Holliday Junction dissolution (dHJ) via the BTR complex. T-loops are typically drawn with strand-invasion promoting a displacement loop. However, this is conjecture, and the t-loop junction identify is unknown.
BLM does many things, including double Holliday Junction dissolution (dHJ) via the BTR complex. T-loops are typically drawn with strand-invasion promoting a displacement loop. However, this is conjecture, and the t-loop junction identify is unknown.
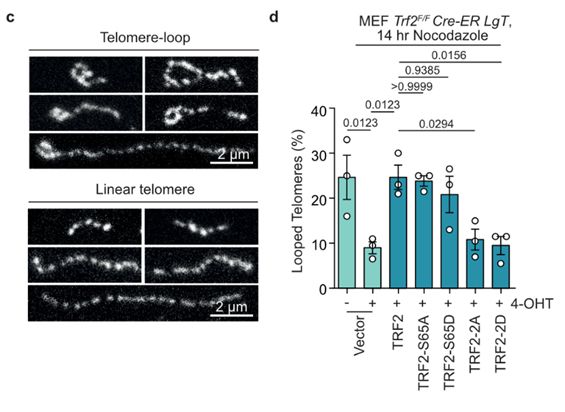
19/Ronnie's Airyscan imaging of telomere macromolecular structure following in situ trioxsalen cross-linking and chromatin spreading suggested TRF2 basic domain phosphorylation promoted the transition from looped to linear telomeres during mitotic arrest, coinciding with telomere DDR induction.
March 17, 2025 at 10:32 AM
19/Ronnie's Airyscan imaging of telomere macromolecular structure following in situ trioxsalen cross-linking and chromatin spreading suggested TRF2 basic domain phosphorylation promoted the transition from looped to linear telomeres during mitotic arrest, coinciding with telomere DDR induction.
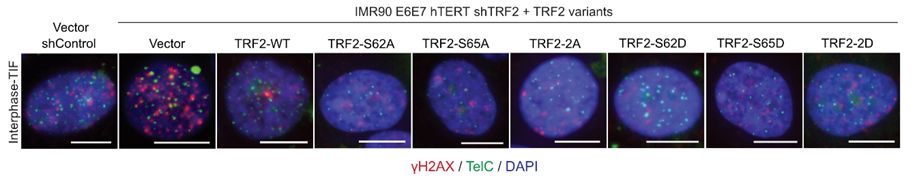
18/Critically, both labs showed that all TRF2 S62 and/or S65 phospo-null or phospho-mimetic mutants retained interphase telomere protection, indicating these residues function specifically in mitotic telomere protection. Diana & Makoto also showed that TRF1 and TRF2 mutants localized to telomeres.
March 17, 2025 at 10:32 AM
18/Critically, both labs showed that all TRF2 S62 and/or S65 phospo-null or phospho-mimetic mutants retained interphase telomere protection, indicating these residues function specifically in mitotic telomere protection. Diana & Makoto also showed that TRF1 and TRF2 mutants localized to telomeres.
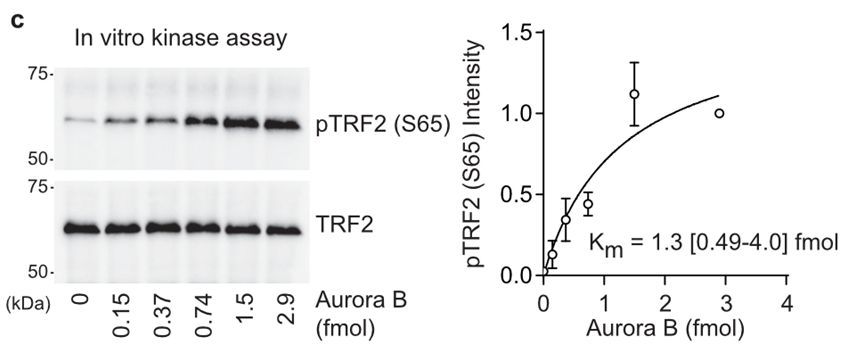
17/Sam created a phosphospecific antibody against pTRF2-S65 and his biochemistry confirmed this residue was phosphorylated specifically during mitotic arrest in an AURKB-dependent fashion.
March 17, 2025 at 10:32 AM
17/Sam created a phosphospecific antibody against pTRF2-S65 and his biochemistry confirmed this residue was phosphorylated specifically during mitotic arrest in an AURKB-dependent fashion.
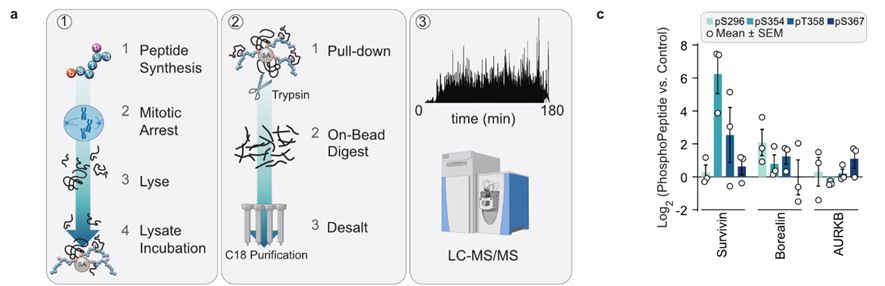
14/Sam used phospho-peptides, mass spec, and biochemistry to identify that the CPC component Survivin bound to phosphorylated pTRF1. Diana’s molecular biology and live imaging confirmed that the CPC factors INCEMP and Survivin are requied for MAD-telomere deprotection.
March 17, 2025 at 10:32 AM
14/Sam used phospho-peptides, mass spec, and biochemistry to identify that the CPC component Survivin bound to phosphorylated pTRF1. Diana’s molecular biology and live imaging confirmed that the CPC factors INCEMP and Survivin are requied for MAD-telomere deprotection.
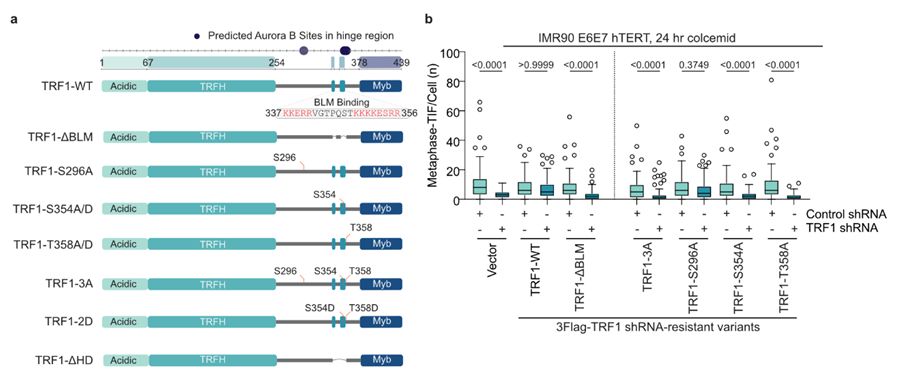
13/Molecular biology by Diana suggested that TRF1 S354 and T358 phosphorylation were required for MAD-telomere deprotection.
Sam created a phospho-specific antibody against pTRF1-T358 and found this residue was phosphorylated specifically during mitotic arrest by AURKB.
Sam created a phospho-specific antibody against pTRF1-T358 and found this residue was phosphorylated specifically during mitotic arrest by AURKB.
March 17, 2025 at 10:32 AM
13/Molecular biology by Diana suggested that TRF1 S354 and T358 phosphorylation were required for MAD-telomere deprotection.
Sam created a phospho-specific antibody against pTRF1-T358 and found this residue was phosphorylated specifically during mitotic arrest by AURKB.
Sam created a phospho-specific antibody against pTRF1-T358 and found this residue was phosphorylated specifically during mitotic arrest by AURKB.
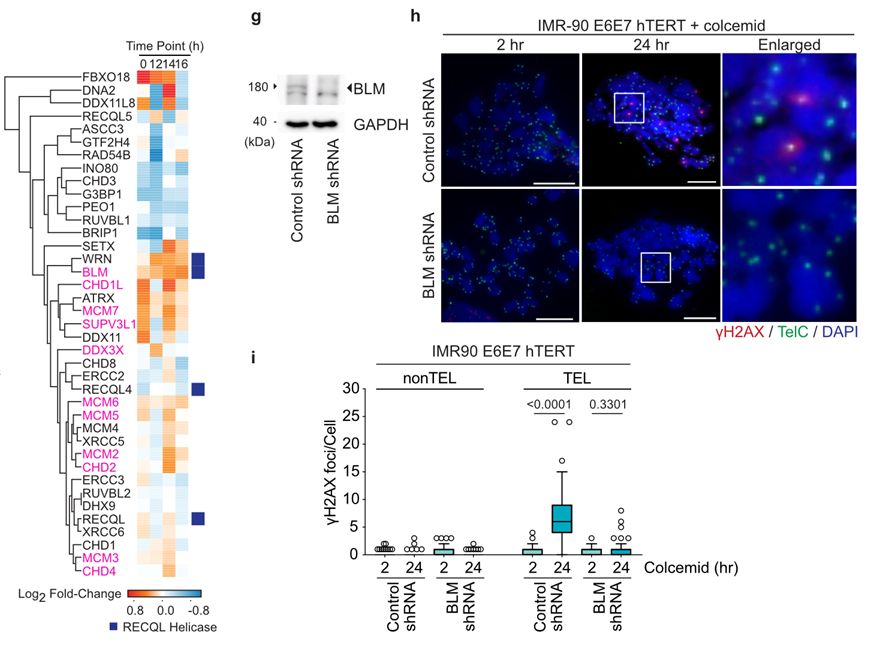
11/To do so, Sam developed an unbiased and time resolved TRF1-APEX2 interactomics pipeline. This revealed that telomeres in mitotically arrested cells interacted with the CPC and BLM helicase. We were excited to find that BLM depletion suppressed MAD-telomere deprotection.
March 17, 2025 at 10:32 AM
11/To do so, Sam developed an unbiased and time resolved TRF1-APEX2 interactomics pipeline. This revealed that telomeres in mitotically arrested cells interacted with the CPC and BLM helicase. We were excited to find that BLM depletion suppressed MAD-telomere deprotection.
9/Telomeres can adopt telomere loops (t-loops) where the distal chromosome end strand-invades the duplex telomeric DNA in cis. Findings from the Cesare, Boulton, and de Lange labs suggest t-loops suppresses DDR activation by sequestering the chromosome terminus, which otherwise is an ATM substrate.

March 17, 2025 at 10:32 AM
9/Telomeres can adopt telomere loops (t-loops) where the distal chromosome end strand-invades the duplex telomeric DNA in cis. Findings from the Cesare, Boulton, and de Lange labs suggest t-loops suppresses DDR activation by sequestering the chromosome terminus, which otherwise is an ATM substrate.
8/ MAD-telomere deprotection does require activity of the mitotic Aurora B kinase (AURKB). It also promotes p53 activation and a minority of mitotic death events during lethal mitotic arrest.
This suggests MAD-telomere deprotection is a regulated signal that alerts cells to mitotic stress.
This suggests MAD-telomere deprotection is a regulated signal that alerts cells to mitotic stress.

March 17, 2025 at 10:32 AM
8/ MAD-telomere deprotection does require activity of the mitotic Aurora B kinase (AURKB). It also promotes p53 activation and a minority of mitotic death events during lethal mitotic arrest.
This suggests MAD-telomere deprotection is a regulated signal that alerts cells to mitotic stress.
This suggests MAD-telomere deprotection is a regulated signal that alerts cells to mitotic stress.
5/Deprotection is the physiological response when chromosome ends lose protection against DNA damage response (DDR) or DNA repair activity despite functional telomere proteins (i.e. shelterin). Canonical telomere deprotection occurs with telomere erosion during ageing.

March 17, 2025 at 10:32 AM
5/Deprotection is the physiological response when chromosome ends lose protection against DNA damage response (DDR) or DNA repair activity despite functional telomere proteins (i.e. shelterin). Canonical telomere deprotection occurs with telomere erosion during ageing.
4/🧵⬇️
TL;DR. During mitotic arrest, Aurora Kinase B of the Chromosome Passenger Complex (CPC) phosphorylates the telomere proteins TRF1 & TRF2, enabling BLM-TOP3A-RMI1/2 to dissolve t-loop junctions and linearize telomeres, promoting localized ATM activation.
(stick around, it’s a cool story!)
TL;DR. During mitotic arrest, Aurora Kinase B of the Chromosome Passenger Complex (CPC) phosphorylates the telomere proteins TRF1 & TRF2, enabling BLM-TOP3A-RMI1/2 to dissolve t-loop junctions and linearize telomeres, promoting localized ATM activation.
(stick around, it’s a cool story!)

March 17, 2025 at 10:32 AM
4/🧵⬇️
TL;DR. During mitotic arrest, Aurora Kinase B of the Chromosome Passenger Complex (CPC) phosphorylates the telomere proteins TRF1 & TRF2, enabling BLM-TOP3A-RMI1/2 to dissolve t-loop junctions and linearize telomeres, promoting localized ATM activation.
(stick around, it’s a cool story!)
TL;DR. During mitotic arrest, Aurora Kinase B of the Chromosome Passenger Complex (CPC) phosphorylates the telomere proteins TRF1 & TRF2, enabling BLM-TOP3A-RMI1/2 to dissolve t-loop junctions and linearize telomeres, promoting localized ATM activation.
(stick around, it’s a cool story!)
Review from our lab out today: "Roles for the 3D Genome In the cell cycle, DNA replication, and double strand break repair." Written by @scientistkate.bsky.social, with her co-supervisor @piptaberlay.bsky.social and collaborator Mat Jones. www.frontiersin.org/journals/cel...
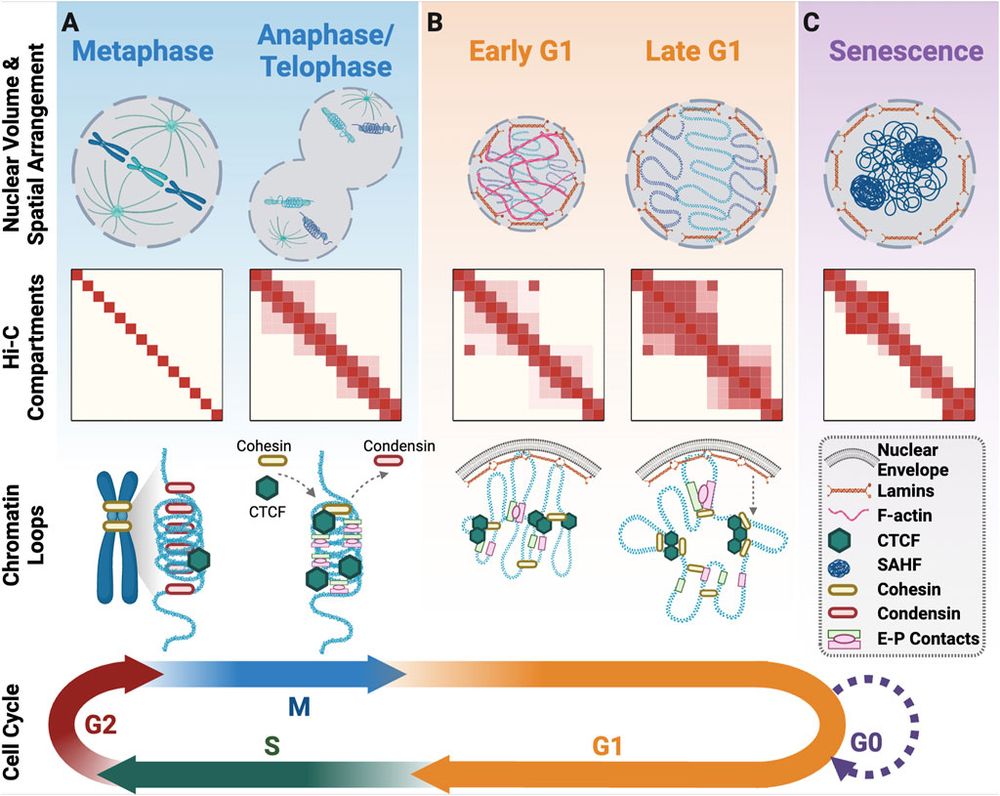
March 4, 2025 at 10:05 PM
Review from our lab out today: "Roles for the 3D Genome In the cell cycle, DNA replication, and double strand break repair." Written by @scientistkate.bsky.social, with her co-supervisor @piptaberlay.bsky.social and collaborator Mat Jones. www.frontiersin.org/journals/cel...
Thank you to Roger Greenberg and Hilda Pickett for organising a fantastic 2025 Mammalian DNA Repair GRC in Ventura, California.
Looking forward to the next time.
Looking forward to the next time.


February 7, 2025 at 12:37 AM
Thank you to Roger Greenberg and Hilda Pickett for organising a fantastic 2025 Mammalian DNA Repair GRC in Ventura, California.
Looking forward to the next time.
Looking forward to the next time.
18/Conversely, targeting NHEJ or SSA promoted HR-dependent mitotic death following IR. This removed damaged cells from the cycling population before chromosome segregation errors could occur. The result was no increase in interferon following ablative IR.

January 13, 2025 at 10:12 AM
18/Conversely, targeting NHEJ or SSA promoted HR-dependent mitotic death following IR. This removed damaged cells from the cycling population before chromosome segregation errors could occur. The result was no increase in interferon following ablative IR.
16/Conversely, the chromosomal structural aberrations generated by NHEJ, MMEJ, and SSA in G1 damaged cells led to chromosome segregation errors and cytosolic nucleic acids. That in turn activated interferon production via cGAS and MAVS, and extrinsic apoptosis via Caspase-8.

January 13, 2025 at 10:12 AM
16/Conversely, the chromosomal structural aberrations generated by NHEJ, MMEJ, and SSA in G1 damaged cells led to chromosome segregation errors and cytosolic nucleic acids. That in turn activated interferon production via cGAS and MAVS, and extrinsic apoptosis via Caspase-8.
13/However, co-targeting HR (RAD51 siRNA) and SSA (RAD52 siRNA) suppressed mitotic death in the G1 irradiated cells. This suggested mitotic death following SSA inhibition stems from G1 damaged cells entering S/G2 and engaging HR, leading to subsequent mitotic death.

January 13, 2025 at 10:12 AM
13/However, co-targeting HR (RAD51 siRNA) and SSA (RAD52 siRNA) suppressed mitotic death in the G1 irradiated cells. This suggested mitotic death following SSA inhibition stems from G1 damaged cells entering S/G2 and engaging HR, leading to subsequent mitotic death.
12/We also targeted the end joining DSB repair pathways: NHEJ (DNA-PKcs, Lig4), MMEJ (POLθ), and SSA (RAD52). Inhibiting any of these pathways promoted death in the first attempted mitosis in G1 irradiated cells that normally survive the first cell division.

January 13, 2025 at 10:12 AM
12/We also targeted the end joining DSB repair pathways: NHEJ (DNA-PKcs, Lig4), MMEJ (POLθ), and SSA (RAD52). Inhibiting any of these pathways promoted death in the first attempted mitosis in G1 irradiated cells that normally survive the first cell division.
11/In agreement, BRCA2 mutant cancer cells deficient for HR (PEO1) were resistant to mitotic arrest and mitotic death following ablative IR. However, spontaneous reversion to WT BRCA2 status (PEO4) simultaneously rescued HR, mitotic arrest, and mitotic death following IR.

January 13, 2025 at 10:12 AM
11/In agreement, BRCA2 mutant cancer cells deficient for HR (PEO1) were resistant to mitotic arrest and mitotic death following ablative IR. However, spontaneous reversion to WT BRCA2 status (PEO4) simultaneously rescued HR, mitotic arrest, and mitotic death following IR.

