We investigated the synthetic pathway and an unexpected and extremely interesting photophysical behaviour.
pubs.rsc.org/en/content/a...

We investigated the synthetic pathway and an unexpected and extremely interesting photophysical behaviour.
pubs.rsc.org/en/content/a...
Triplet state reactivity and long-lived room temperature phosphorescence of chalcones enabled by micelles | ChemRxiv - doi.org/10.26434/che...

Triplet state reactivity and long-lived room temperature phosphorescence of chalcones enabled by micelles | ChemRxiv - doi.org/10.26434/che...
doi.org/10.1039/D4GC...

doi.org/10.1039/D4GC...
Just published in the Journal of the Americal Chemical Society
pubs.acs.org/doi/10.1021/...
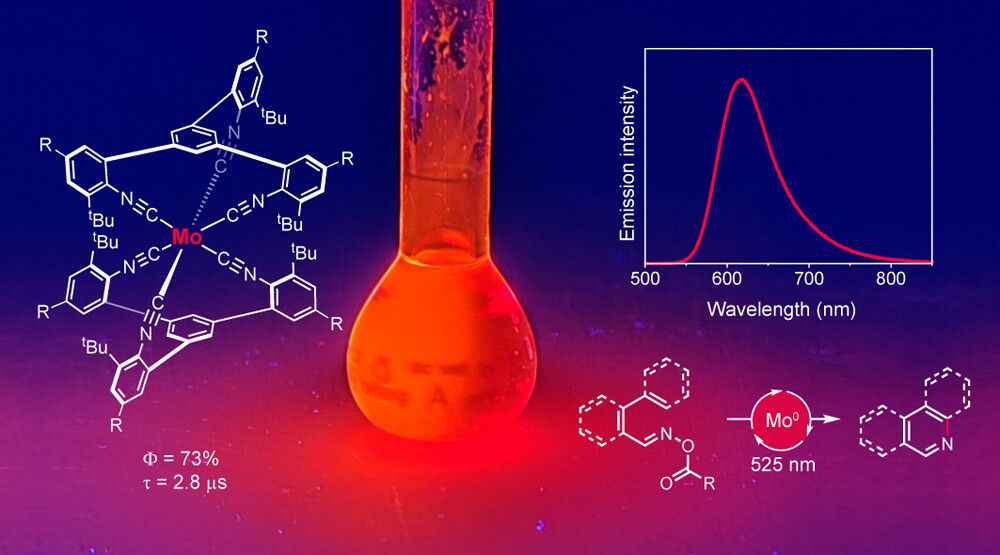
Just published in the Journal of the Americal Chemical Society
pubs.acs.org/doi/10.1021/...
Our perspective just published in JACS Au:
pubs.acs.org/doi/10.1021/...
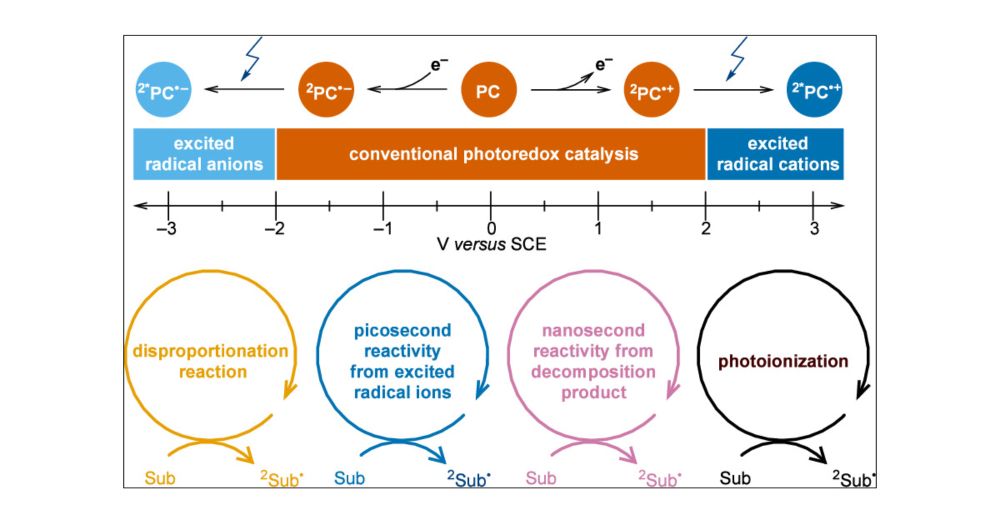
Our perspective just published in JACS Au:
pubs.acs.org/doi/10.1021/...
Check out how our team realized the mechanism with no apparent absorption in the "normal" UV-vis spectrum. Spearheaded by Julian and Martin!

Check out how our team realized the mechanism with no apparent absorption in the "normal" UV-vis spectrum. Spearheaded by Julian and Martin!

doi.org/10.1002/anie...

doi.org/10.1002/anie...

