
Stefan Borsley
@stefanborsley.bsky.social
Nonequilibrium chemistry. Royal Society University Research Fellow. Durham Chemistry. Occasionally I travel too.
Enjoyed the last week in Wrocław, where @szyszkobart.bsky.social organised a fantastic supramolecular symposium. Excellent hospitality and great to spend time with the other speakers @screspi.bsky.social @annajmcconnell.bsky.social @bmschmidtlab.bsky.social and Jen Hiscock. Thanks for a great week!

September 28, 2025 at 11:16 AM
Enjoyed the last week in Wrocław, where @szyszkobart.bsky.social organised a fantastic supramolecular symposium. Excellent hospitality and great to spend time with the other speakers @screspi.bsky.social @annajmcconnell.bsky.social @bmschmidtlab.bsky.social and Jen Hiscock. Thanks for a great week!
Reposted by Stefan Borsley
Can you work out how fast a molecular motor rotates from in situ measurments? Turns out that yes, you can, as we show today in @jacs.acspublications.org
pubs.acs.org/doi/10.1021/...
@profdaveleigh.bsky.social @stefanborsley.bsky.social
pubs.acs.org/doi/10.1021/...
@profdaveleigh.bsky.social @stefanborsley.bsky.social
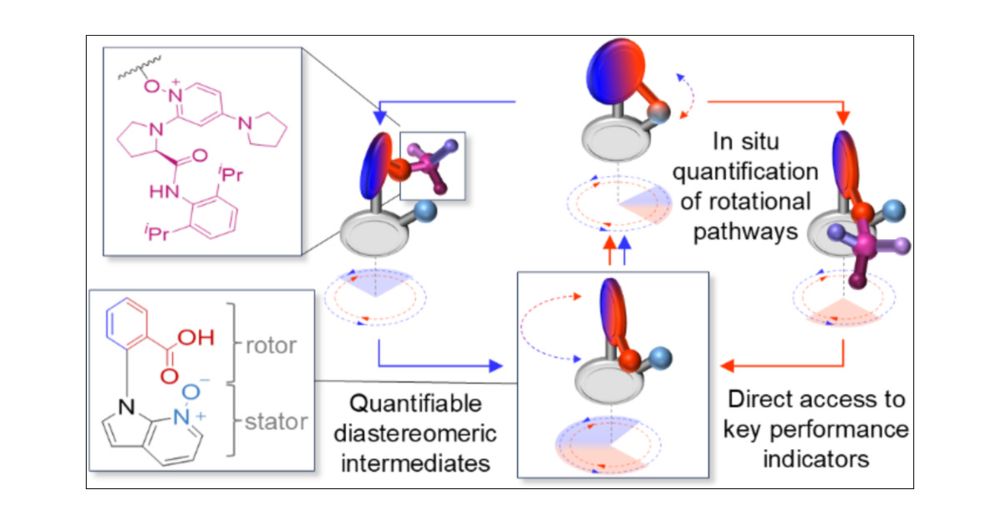
In Situ Quantification of Directional Rotation by a Catalysis-Driven Azaindole-N-Oxide–Phenoic Acid Molecular Motor
We report the in situ quantification of directional rotation of a new type of catalysis-driven rotary motor featuring a phenyl carboxylic acid rotor attached to a 7-azaindole-N-oxide stator through a biaryl C–N bond. Continuous directional rotation of the rotor about the stator is driven by the achiral motor’s rotary catalysis of carbodiimide hydration in the presence of a chiral pyrrolidinylpyridine-N-oxide. The catalytic cycle features an intermediate O-acyl-azaindole-N-oxide ester tether formed between the carboxylic acid of the rotor and the N-oxide of the stator. Face-selective cleavage of the tether by the chiral pyrrolidinylpyridine-N-oxide additive generates relatively long-lived diastereomeric pyridine-N-oxide esters of the phenyl carboxylic acid. These are hydrolyzed during the catalytic cycle to reform the carboxylic acid resting state of the motor, completing net directional 360° rotation. In contrast to previous catalysis-driven motor-molecules, the motor’s directionality could be determined directly from the transient concentrations of the diastereomeric intermediates formed during rotary catalysis. This avoids reliance on restricted rotation models to assess motor directionality and provides direct access to other key performance indicators such as motor speed and catalytic, coupling and fuel efficiency. The in situ-determined directionality of the motor was found to be in excellent agreement with the directionality determined from a restricted rotation model, supporting both the efficacy of the new approach and the validity of using appropriately designed restricted rotation models. The results establish a straightforward method for the in situ quantification of various aspects of motor behavior, aiding the design and optimization of artificial molecular motors.
pubs.acs.org
August 1, 2025 at 9:21 AM
Can you work out how fast a molecular motor rotates from in situ measurments? Turns out that yes, you can, as we show today in @jacs.acspublications.org
pubs.acs.org/doi/10.1021/...
@profdaveleigh.bsky.social @stefanborsley.bsky.social
pubs.acs.org/doi/10.1021/...
@profdaveleigh.bsky.social @stefanborsley.bsky.social
Great to have @benjaminoacid.bsky.social (University of Padova) visiting Durham this weekend. Ben delivered a fantastic workshop on kinetic analysis yesterday. Lots of science (and other) discussions over a barbecue today!

July 5, 2025 at 2:45 PM
Great to have @benjaminoacid.bsky.social (University of Padova) visiting Durham this weekend. Ben delivered a fantastic workshop on kinetic analysis yesterday. Lots of science (and other) discussions over a barbecue today!
Thanks to the organisers for an excellent conference ar #ISMSC2025. Thoroughly enjoyed some excellent science, meeting lots of new people and catching up with old friends. Thanks also for the opportunity for a talk to share some of the work we are up to in my group. See more at borslab.net.




May 30, 2025 at 12:58 PM
Thanks to the organisers for an excellent conference ar #ISMSC2025. Thoroughly enjoyed some excellent science, meeting lots of new people and catching up with old friends. Thanks also for the opportunity for a talk to share some of the work we are up to in my group. See more at borslab.net.
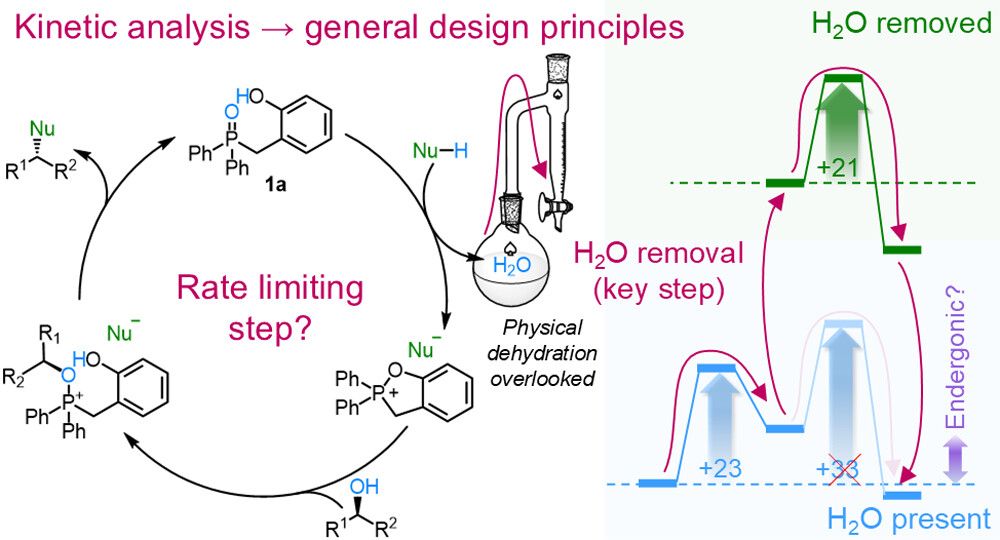
An argument over a whisky turned into a paper! We apply kinetic modelling to describe Denton’s catalytic Mitsunobu reaction, and show how it can be improved! Not a paper I ever expected to write, but a testament to the benefits of chatting science with friends! Thanks @keithandrews.bsky.social!
How can Le Chatelier drive kinetics? @stefanborsley.bsky.social and I analyse Denton's organocatalytic Mitsunobu reaction and show water removal improvements outperform catalyst redesigns. Why? Removing water part way through the reaction resets the potential energy surface! doi.org/10.1021/jacs...
May 13, 2025 at 7:17 AM
An argument over a whisky turned into a paper! We apply kinetic modelling to describe Denton’s catalytic Mitsunobu reaction, and show how it can be improved! Not a paper I ever expected to write, but a testament to the benefits of chatting science with friends! Thanks @keithandrews.bsky.social!
Reposted by Stefan Borsley
Check out how we made a minuscule motor rotate 24 times in one direction on its own! Huge thanks to all involved, especially @profdaveleigh.bsky.social for making this work possible
@pubs.acs.org
Huakui, Axel, @stefanborsley.bsky.social @benjaminoacid.bsky.social & Alex
pubs.acs.org/doi/10.1021/...
@pubs.acs.org
Huakui, Axel, @stefanborsley.bsky.social @benjaminoacid.bsky.social & Alex
pubs.acs.org/doi/10.1021/...

Structural Influence of the Chemical Fueling System on a Catalysis-Driven Rotary Molecular Motor
Continuous directionally biased 360° rotation about a covalent single bond was recently realized in the form of a chemically fueled 1-phenylpyrrole 2,2′-dicarboxylic acid rotary molecular motor. However, the original fueling system and reaction conditions resulted in a motor directionality of only ∼3:1 (i.e., on average a backward rotation for every three forward rotations), along with a catalytic efficiency for the motor operation of 97% and a fuel efficiency of 14%. Here, we report on the efficacy of a series of chiral carbodiimide fuels and chiral hydrolysis promoters (pyridine and pyridine N-oxide derivatives) in driving improved directional rotation of this motor-molecule. We outline the complete reaction network for motor operation, composed of directional, futile, and slip cycles. Using derivatives of the motor where the final conformational step in the 360° rotation is either very slow or completely blocked, the phenylpyrrole diacid becomes enantiomerically enriched, allowing the kinetic gating of the individual steps in the catalytic cycle to be measured. The chiral carbodiimide fuel that produces the highest directionality gives 13% enantiomeric excess (e.e.) for the anhydride-forming kinetically gated step, while the most effective chiral hydrolysis promoter generates 90% e.e. for the kinetically gated hydrolysis step. Combining the best-performing fuel and hydrolysis promoter into a single fueling system results in a 92% e.e.. Under a dilute chemostated fueling regime (to avoid N-acyl urea formation at high carbodiimide concentrations with pyridine N-oxide hydrolysis promoters), the motor continuously rotates with a directionality of ∼24:1 (i.e., a backward rotation for every 24 forward rotations) with a catalytic efficiency of >99% and a fuel efficiency of 51%.
pubs.acs.org
February 28, 2025 at 9:39 AM
Check out how we made a minuscule motor rotate 24 times in one direction on its own! Huge thanks to all involved, especially @profdaveleigh.bsky.social for making this work possible
@pubs.acs.org
Huakui, Axel, @stefanborsley.bsky.social @benjaminoacid.bsky.social & Alex
pubs.acs.org/doi/10.1021/...
@pubs.acs.org
Huakui, Axel, @stefanborsley.bsky.social @benjaminoacid.bsky.social & Alex
pubs.acs.org/doi/10.1021/...
Congrats, Ben!
This will be a fantastic PhD opportunity for whoever is lucky enough to get it! Can… I apply…?
This will be a fantastic PhD opportunity for whoever is lucky enough to get it! Can… I apply…?
I’m excited to share that I will be moving to the University of Ulm as a junior group leader and I have a fully-funded open PhD position co-supervised by me and Max von Delius @mvdelius.bsky.social . Apply at benjamin.roberts@unipd.it, any shares would be appreciated!
www.bmwrlab.com/work-with-us
www.bmwrlab.com/work-with-us
Work with us | The Roberts Lab
www.bmwrlab.com
February 26, 2025 at 7:23 AM
Congrats, Ben!
This will be a fantastic PhD opportunity for whoever is lucky enough to get it! Can… I apply…?
This will be a fantastic PhD opportunity for whoever is lucky enough to get it! Can… I apply…?
Reposted by Stefan Borsley
Come and join my new group! We have two funded PhD positions available in the areas of supramolecular chemistry and polymer nanotechnology. See fieldengroup.net for more details! Reposts much appreciated!
Fielden Group - Nanotechnology Research
We're an interdisciplinary research group led by Dr Stephen Fielden within the School of Chemistry at the
University of Birmingham. We research polymers, nanoparticles and supramolecular chemistry.
fieldengroup.net
January 15, 2025 at 3:38 PM
Come and join my new group! We have two funded PhD positions available in the areas of supramolecular chemistry and polymer nanotechnology. See fieldengroup.net for more details! Reposts much appreciated!
A really fun project, delivered on the back of Peng-Lai’s fantastic efforts, ably assisted by Martin and of course our collaborators in Strasbourg, Alessandro and @giusepponelab.bsky.social, who taught us lots of polymer and materials chemistry.
Thanks all!
Thanks all!
Peng-Lai, @stefanborsley.bsky.social, Martin & our collaborators Alessandro and @giusepponelab.bsky.social demonstrate how a catalyst transduces chemical energy to perform mechanical work in www.nature.com/articles/s41... in @nature.com. tinyurl.com/jny7nen5. Animation @scicommstudios.bsky.social😀
January 15, 2025 at 5:25 PM
A really fun project, delivered on the back of Peng-Lai’s fantastic efforts, ably assisted by Martin and of course our collaborators in Strasbourg, Alessandro and @giusepponelab.bsky.social, who taught us lots of polymer and materials chemistry.
Thanks all!
Thanks all!
Great to see our research group taking shape with Martha, Zac and Willow (left to right) taking the plunge and joining me for what I hope will be some fun chemistry.
More about the group on our website: www.borslab.net/meet-the-gro...
More about the group on our website: www.borslab.net/meet-the-gro...

November 13, 2024 at 2:26 PM
Great to see our research group taking shape with Martha, Zac and Willow (left to right) taking the plunge and joining me for what I hope will be some fun chemistry.
More about the group on our website: www.borslab.net/meet-the-gro...
More about the group on our website: www.borslab.net/meet-the-gro...
Check out our review on ‘Ratcheting Synthesis’: www.nature.com/articles/s41...
We discuss ratchet mechanisms in a wide range of fields. Written with James Gallagher, Ben Roberts and Dave Leigh.
We discuss ratchet mechanisms in a wide range of fields. Written with James Gallagher, Ben Roberts and Dave Leigh.

December 17, 2023 at 11:00 AM
Check out our review on ‘Ratcheting Synthesis’: www.nature.com/articles/s41...
We discuss ratchet mechanisms in a wide range of fields. Written with James Gallagher, Ben Roberts and Dave Leigh.
We discuss ratchet mechanisms in a wide range of fields. Written with James Gallagher, Ben Roberts and Dave Leigh.



