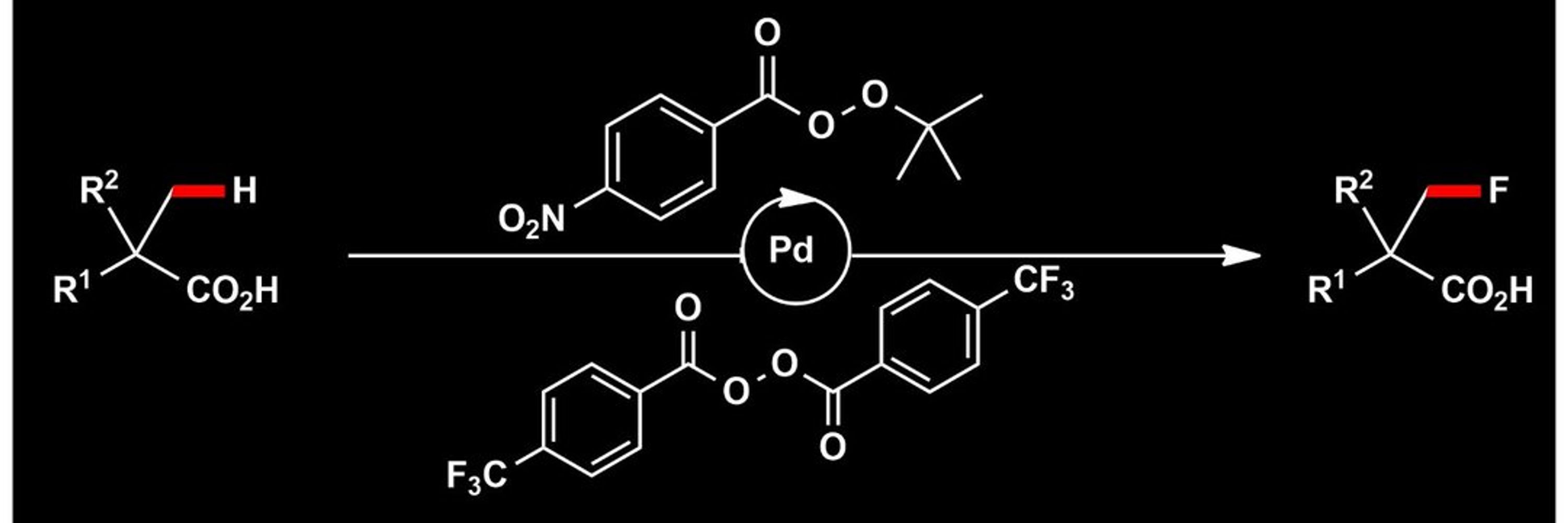
Catalysis | Glycochemistry | C–H Activation
doi.org/10.1002/anie...
#chemsky

doi.org/10.1002/anie...
#chemsky
#SuFEx
👉 buff.ly/hjkyEap

#SuFEx
👉 buff.ly/hjkyEap
'Molecular Stitching' approach, enabling one-step access to a diverse library of γ-alkylidene lactones, is now out in @chemicalscience.rsc.org
#MyFirstChemSci
Link to the full article: doi.org/10.1039/D5SC...

'Molecular Stitching' approach, enabling one-step access to a diverse library of γ-alkylidene lactones, is now out in @chemicalscience.rsc.org
#MyFirstChemSci
Link to the full article: doi.org/10.1039/D5SC...
www.uni-kiel.de/de/detailans...

www.uni-kiel.de/de/detailans...
doi.org/10.1039/D5SC...

doi.org/10.1039/D5SC...
pubs.acs.org/doi/10.1021/...
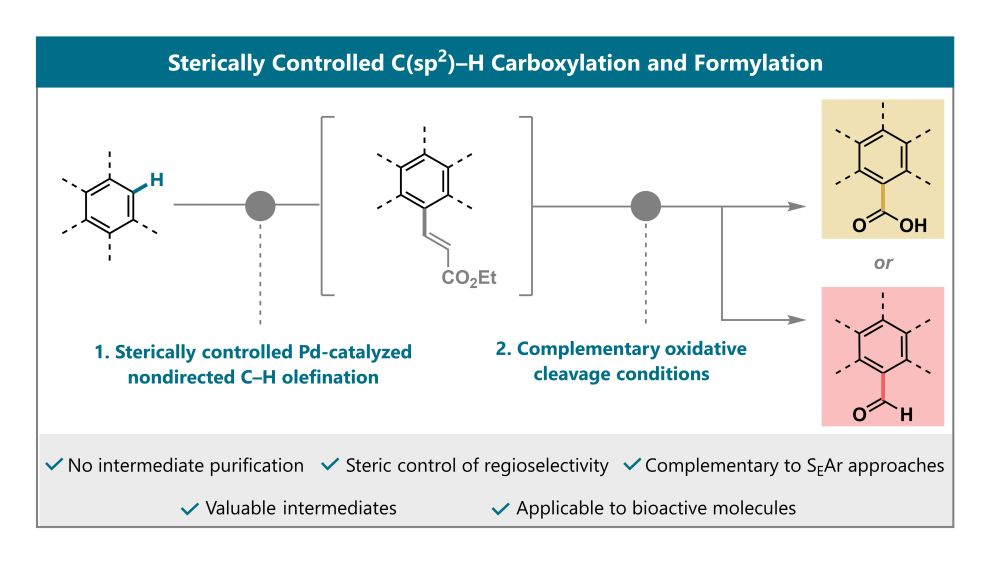
chemrxiv.org/engage/chemr... #chemsky #chemistry

chemrxiv.org/engage/chemr... #chemsky #chemistry


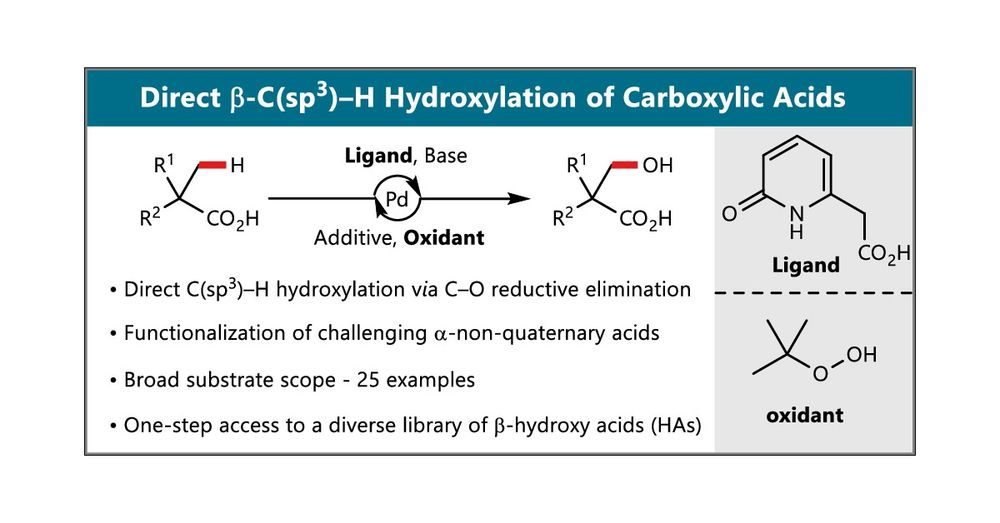
@sourjyamal.bsky.social, who - after a more than half-year publication-process-odyssey - have published their method on the gamma-lactonization of carboxylic acids, including highly challenging beta-non-quaternary substrates doi.org/10.1021/acsc...


www.uni-kiel.de/en/details/n...

www.uni-kiel.de/en/details/n...

