
What a diwali 😀

What a diwali 😀
How does the fusion domain engage and insert into the host cell membrane? How is the fusion harpoon stabilized? How is the harpoon stored before it can be deployed?
pubmed.ncbi.nlm.nih.gov/40555792/

How does the fusion domain engage and insert into the host cell membrane? How is the fusion harpoon stabilized? How is the harpoon stored before it can be deployed?
pubmed.ncbi.nlm.nih.gov/40555792/
Roghly $1 bn of the total science scholarly publishing market $28 bn
Roghly $1 bn of the total science scholarly publishing market $28 bn
To me, the most important are:
Read often, read broadly (incl. older papers and outside your field), and learn to read some papers in detail and others more superficially (and quickly)
The outcome just got published🧵👇
Sequence of the SARS-CoV-2 Spike Transmembrane Domain Encodes Conformational Dynamics
pubs.acs.org/doi/10.1021/...

The outcome just got published🧵👇
Sequence of the SARS-CoV-2 Spike Transmembrane Domain Encodes Conformational Dynamics
pubs.acs.org/doi/10.1021/...

BlueSky user numbers have hit a new record high in recent days, while the number of people deleting their accounts on X/Twitter has rocketed 🚀
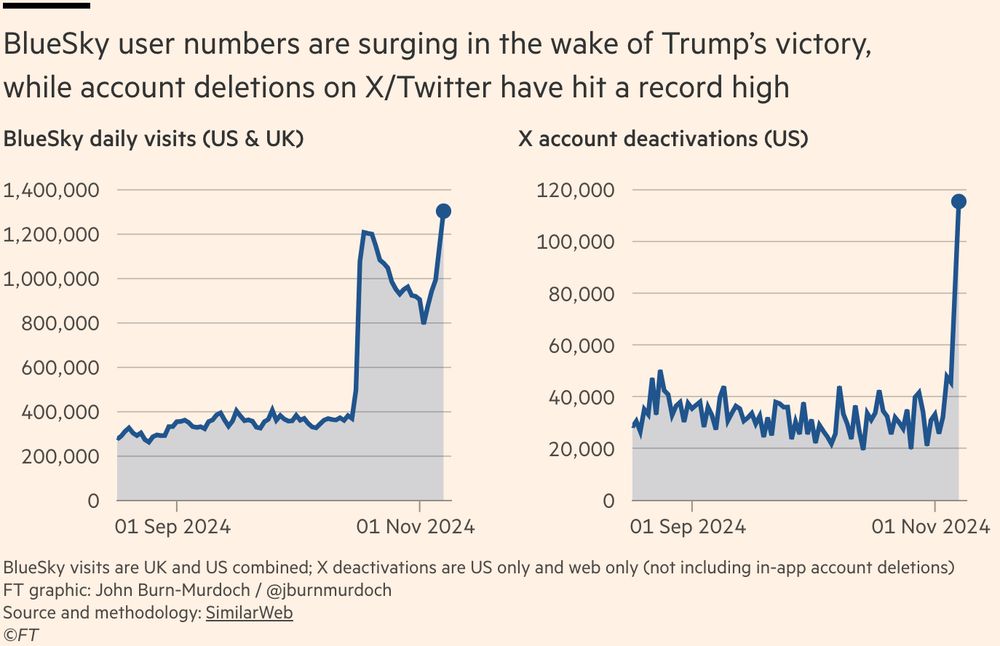
The recent events in the US were a big nail in the ⚰️, even if not the last.
The recent events in the US were a big nail in the ⚰️, even if not the last.


