
Perhaps misfolded RNAs — not just amyloid proteins — are the central structural entity driving disease.

Perhaps misfolded RNAs — not just amyloid proteins — are the central structural entity driving disease.
Two potential exits:
Active reset: when conditions normalize, condensates dissolve, RBPs shift, RNA refolds.
Passive fade: cells with stress-memory may grow slower, and are outcompeted over generations.

Two potential exits:
Active reset: when conditions normalize, condensates dissolve, RBPs shift, RNA refolds.
Passive fade: cells with stress-memory may grow slower, and are outcompeted over generations.
Newly folded RNAs bind stress-linked RBPs to assemble new condensates, passed on during cell division.
Even daughter cells never facing the original stress can inherit the RNA structural memory – and its stress-adapted traits.

Newly folded RNAs bind stress-linked RBPs to assemble new condensates, passed on during cell division.
Even daughter cells never facing the original stress can inherit the RNA structural memory – and its stress-adapted traits.
Some RNAs can act as both template & catalyst, guiding new RNAs with the same sequence to fold the same way.
RNA alone is unstable, but RBPs stabilize the template, & condensates boost RNA–RNA interactions—as a microreaction chamber
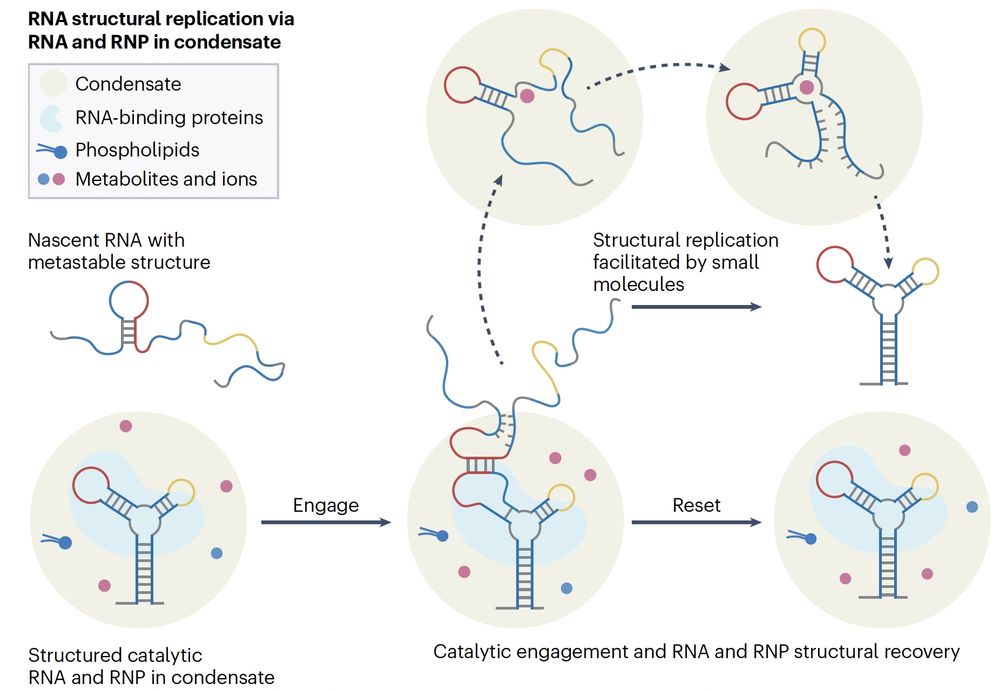
Some RNAs can act as both template & catalyst, guiding new RNAs with the same sequence to fold the same way.
RNA alone is unstable, but RBPs stabilize the template, & condensates boost RNA–RNA interactions—as a microreaction chamber
Under stress, RNA can adopt a new shape—and if RNA-binding proteins “lock” it in, that shape becomes a molecular memory of the stress.
Think Waddington’s epigenetic landscape—but for RNA folding energy valleys.

Under stress, RNA can adopt a new shape—and if RNA-binding proteins “lock” it in, that shape becomes a molecular memory of the stress.
Think Waddington’s epigenetic landscape—but for RNA folding energy valleys.
rdcu.be/eBwPJ

rdcu.be/eBwPJ


These sncRNAs go beyond RNAi, adopting aptamer-like roles by binding TLR7/8 to shape immune responses
www.jbc.org/article/S002...
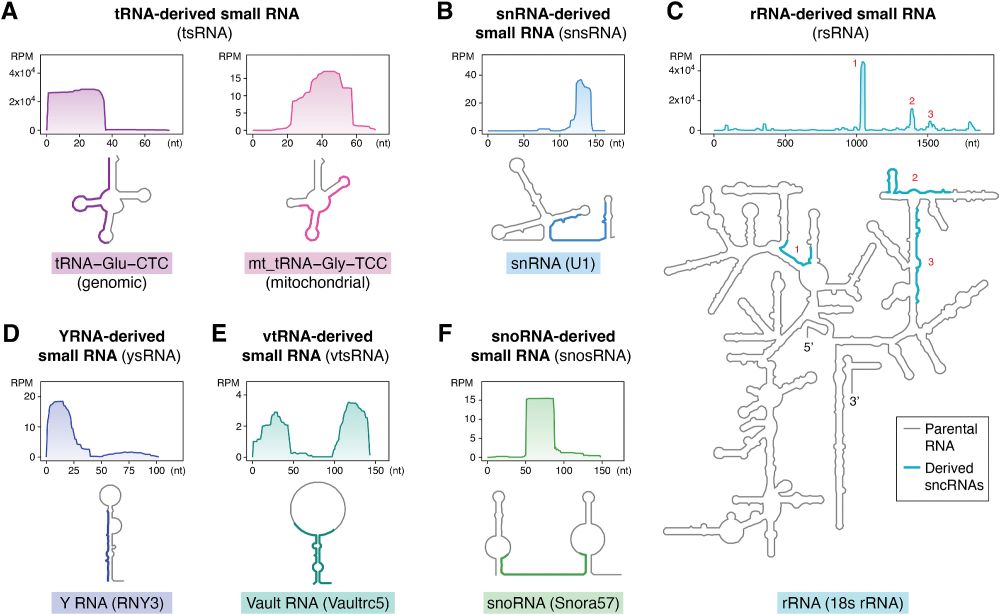
These sncRNAs go beyond RNAi, adopting aptamer-like roles by binding TLR7/8 to shape immune responses
www.jbc.org/article/S002...
Uncovering these diverse triggers may pave new paths for preventing/treating autoimmune diseases.
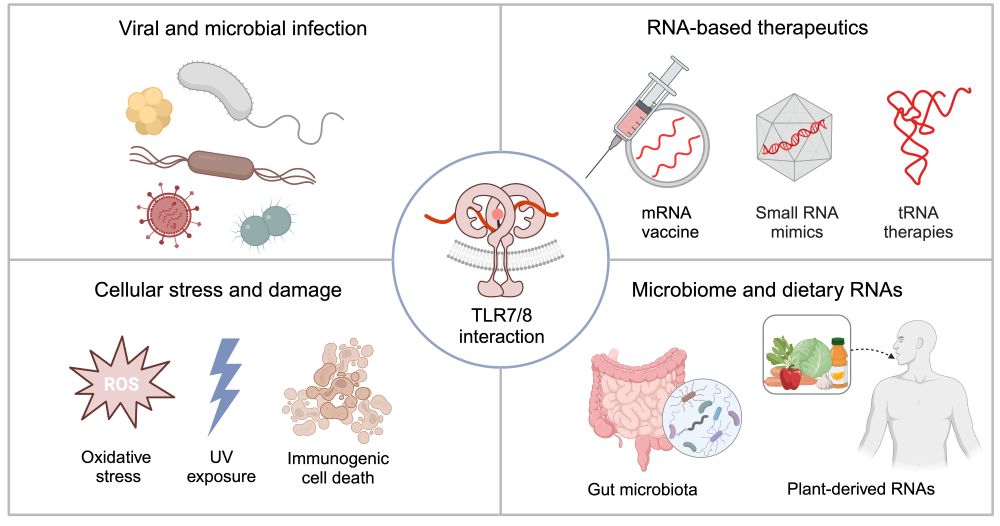
Uncovering these diverse triggers may pave new paths for preventing/treating autoimmune diseases.
www.cell.com/trends/bioch...
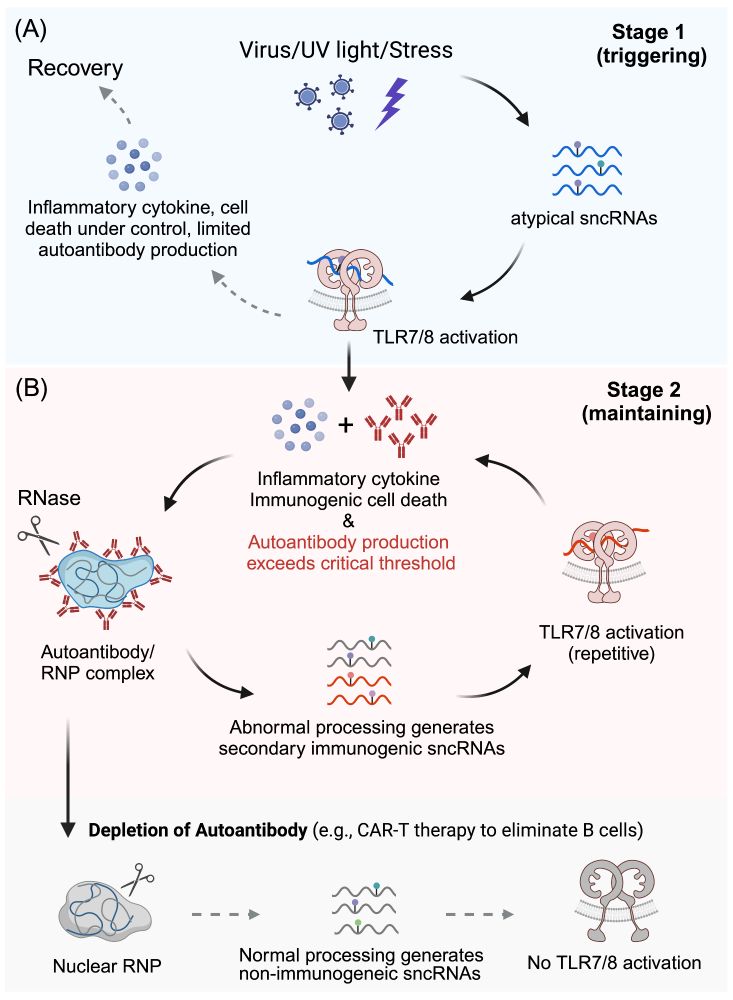
www.cell.com/trends/bioch...





