
TheLewisLab.net
Read more in our preprint: tinyurl.com/4yrwftrn

Read more in our preprint: tinyurl.com/4yrwftrn



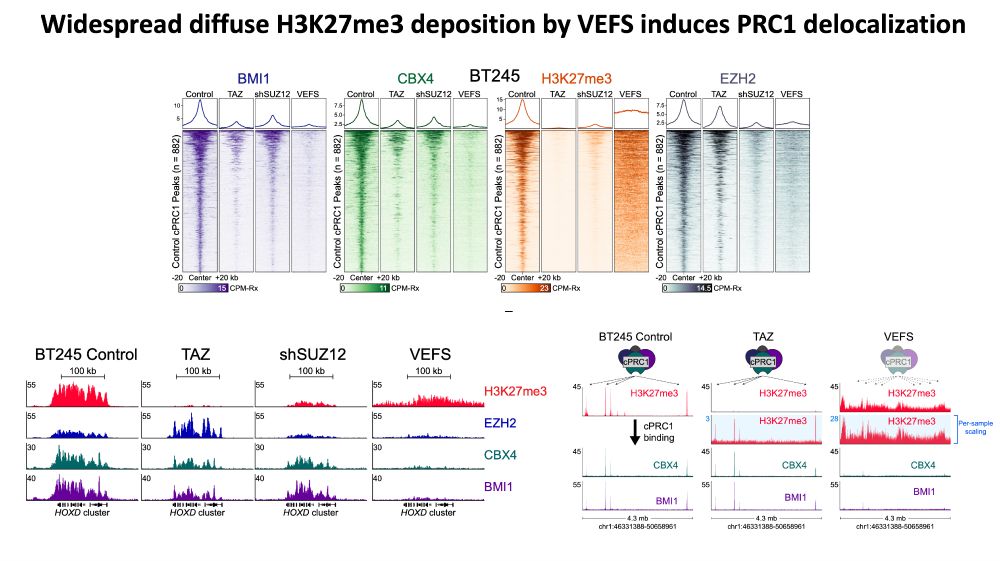
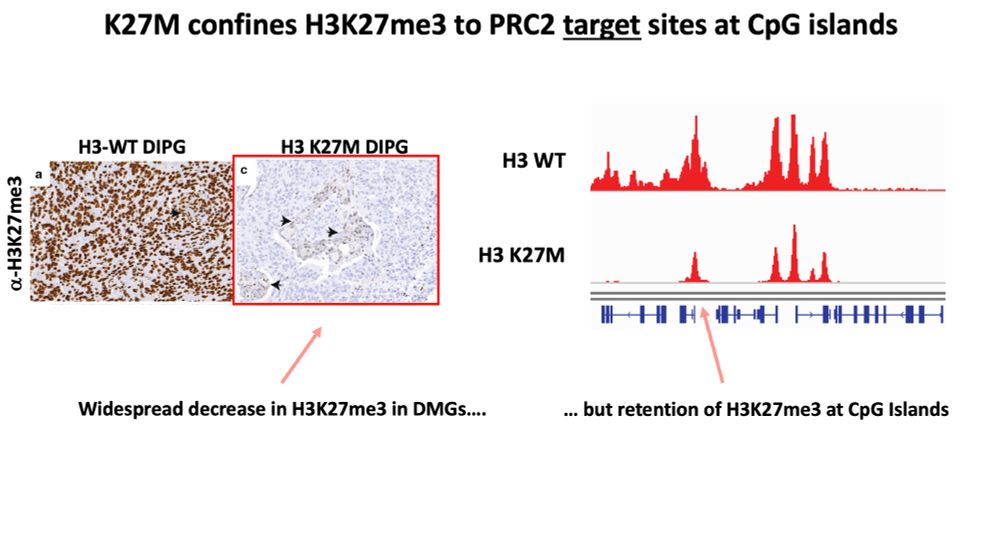
Result: diffuse H3K27me3 that pulls PRC1 away from its normal targets, disrupting gene silencing.
Read more in our preprint: tinyurl.com/4yrwftrn

Result: diffuse H3K27me3 that pulls PRC1 away from its normal targets, disrupting gene silencing.
Read more in our preprint: tinyurl.com/4yrwftrn

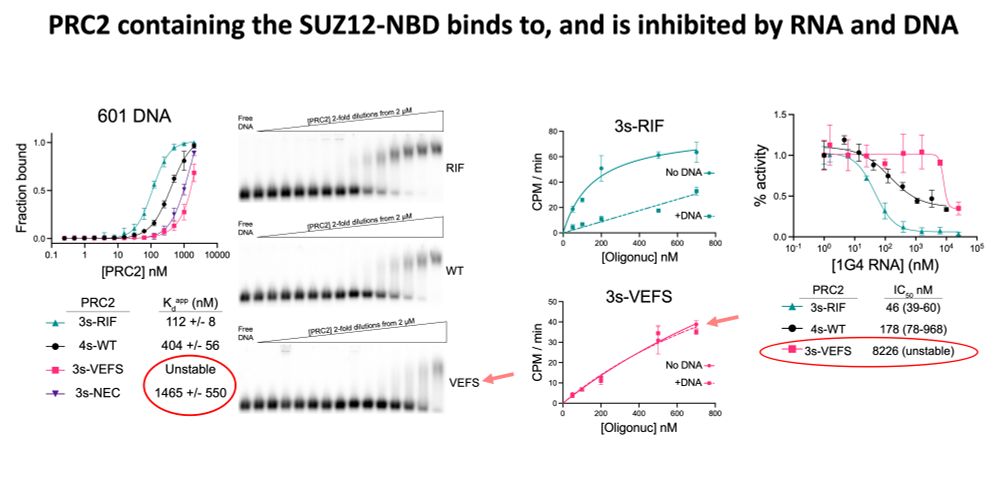
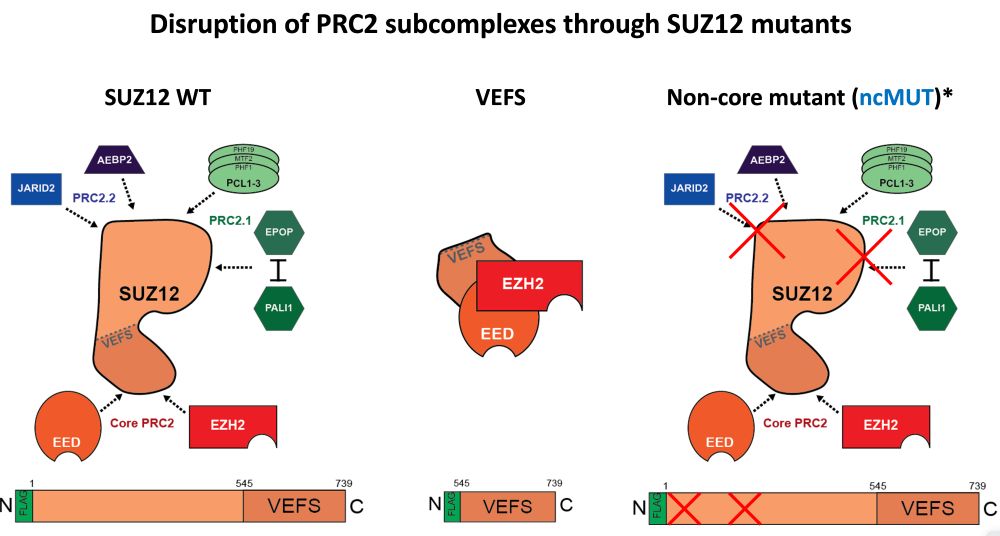
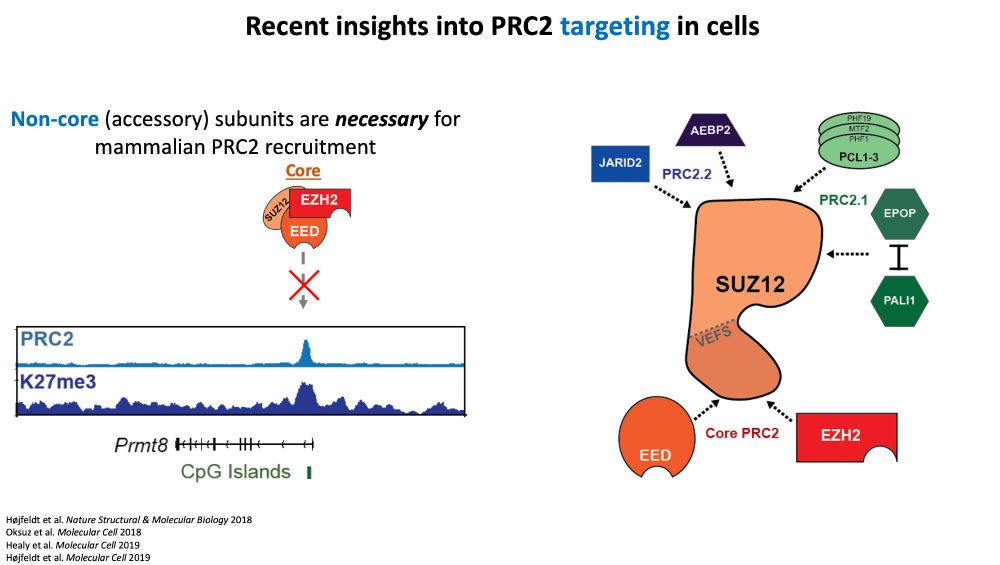
Result: strong H3K27me3 at intended sites, little elsewhere. Arrow size = rate. Bubble size = how much PRC2 is in that state.

Result: strong H3K27me3 at intended sites, little elsewhere. Arrow size = rate. Bubble size = how much PRC2 is in that state.
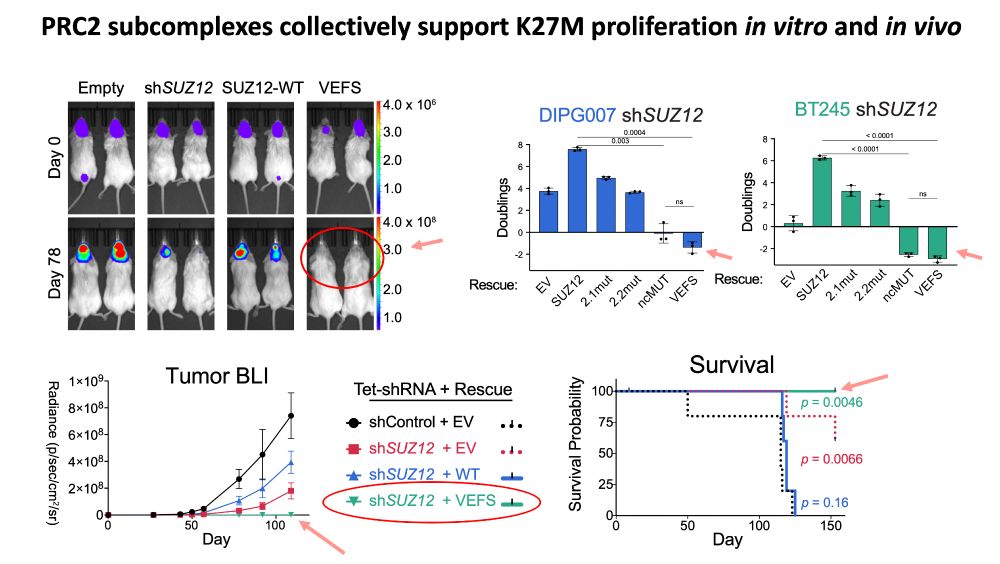
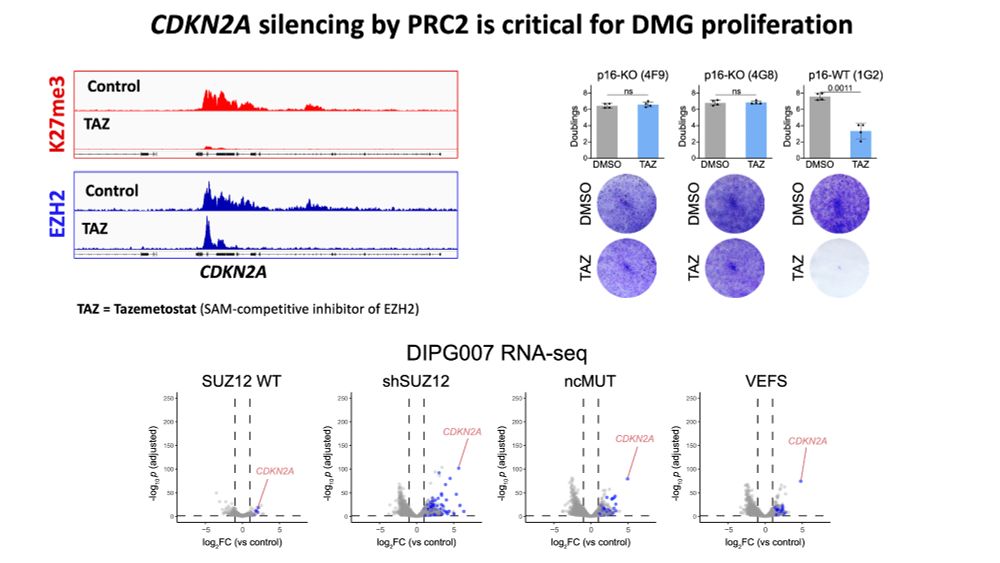
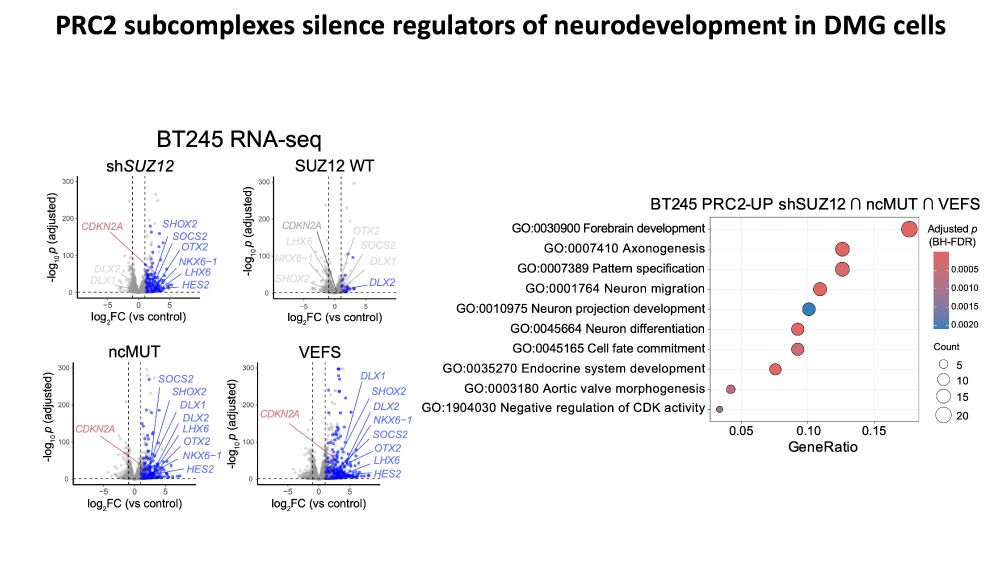
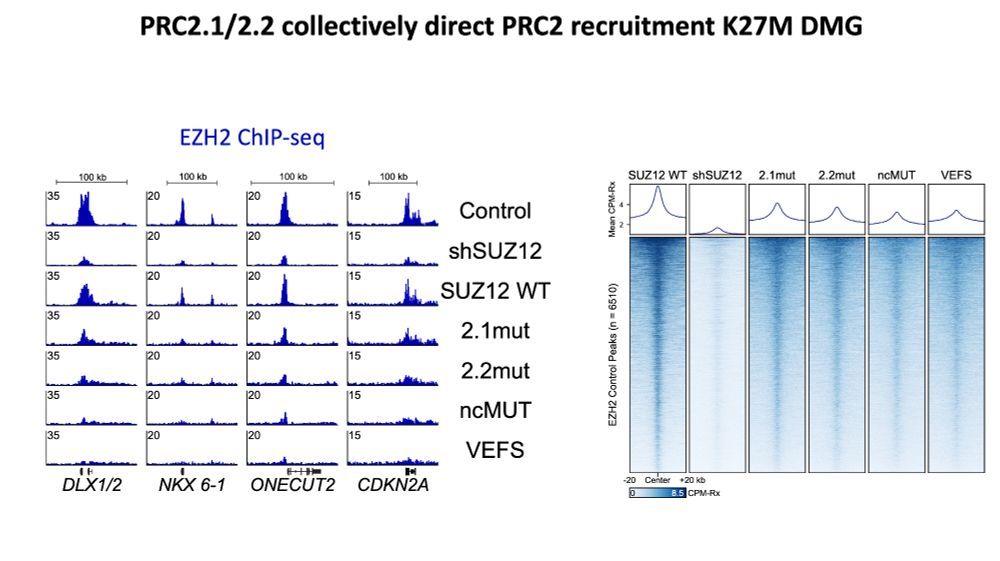
www.biorxiv.org/content/10.1...

www.biorxiv.org/content/10.1...






