
Two catalysts were found to be effective in peracid oxidations of alcohols: TEMPO with Bu4NBr or TMP-HBr.

Two catalysts were found to be effective in peracid oxidations of alcohols: TEMPO with Bu4NBr or TMP-HBr.
A C2-symmetric borinic acid can achieve highly enantioselective desymmetrization of 2,2-disubstituted-1,3-propanediols

A C2-symmetric borinic acid can achieve highly enantioselective desymmetrization of 2,2-disubstituted-1,3-propanediols
Planar chiral [2.2]paracyclophane-based isothiourea catalysts catalyze a highly efficient enantioselective fluorination of carboxylic acids

Planar chiral [2.2]paracyclophane-based isothiourea catalysts catalyze a highly efficient enantioselective fluorination of carboxylic acids
Superacid-catalyzed Pictet-Spengler reactions

Superacid-catalyzed Pictet-Spengler reactions
Pd-catalyzed alkene carboamination and carboalkoxylation reactions of a wide range of (hetero)arylthianthrenium triflates

Pd-catalyzed alkene carboamination and carboalkoxylation reactions of a wide range of (hetero)arylthianthrenium triflates
A three-component reaction of β-ketonitriles, carbonyl- and semistabilized pyridinium ylide precursors, and aldehydes provides trans-4,5-dihydrofuran-3-carbonitriles

A three-component reaction of β-ketonitriles, carbonyl- and semistabilized pyridinium ylide precursors, and aldehydes provides trans-4,5-dihydrofuran-3-carbonitriles
Douglass features The Zhang/Yang Synthesis of Janthinoid A: Zhong-Chao Zhang and Zhen Yang of Peking University Shenzhen Graduate School assembled 3 by way of the oxidative cyclization of the diester 1 to the lactone 2.
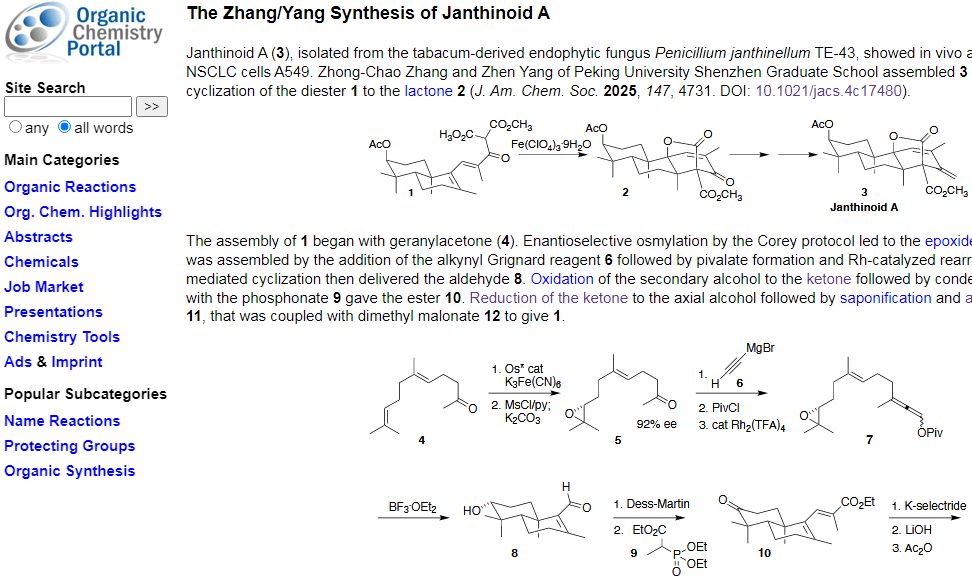
Douglass features The Zhang/Yang Synthesis of Janthinoid A: Zhong-Chao Zhang and Zhen Yang of Peking University Shenzhen Graduate School assembled 3 by way of the oxidative cyclization of the diester 1 to the lactone 2.
Janthinoid A, isolated from the tabacum-derived endophytic fungus Penicillium janthinellum TE-43, showed in vivo anti-tumor activity against NSCLC cells A549.
More Info: www.organic-chemistry.org/Highlights/2...

Janthinoid A, isolated from the tabacum-derived endophytic fungus Penicillium janthinellum TE-43, showed in vivo anti-tumor activity against NSCLC cells A549.
More Info: www.organic-chemistry.org/Highlights/2...
tert-Butanesulfinyl imines can be used for asymmetric syntheses of β-substituted as well as β,β- and α,β-disubstituted β-amino acids

tert-Butanesulfinyl imines can be used for asymmetric syntheses of β-substituted as well as β,β- and α,β-disubstituted β-amino acids
The combination of Ph3P/ICH2CH2I promotes a dehydroxylative sulfonylation of benzylic and allylic alcohols with a variety of sulfinates

The combination of Ph3P/ICH2CH2I promotes a dehydroxylative sulfonylation of benzylic and allylic alcohols with a variety of sulfinates
A formal (3+2)-cycloaddition of donor-acceptor cyclopropanes and ammonium thiocyanate

A formal (3+2)-cycloaddition of donor-acceptor cyclopropanes and ammonium thiocyanate
A useful catalyst system for the Heck coupling of aryl chlorides has been developed. The choice of ligand is critical for activity.

A useful catalyst system for the Heck coupling of aryl chlorides has been developed. The choice of ligand is critical for activity.
A catalyst-free synthesis of phosphinic amides from hydroxyl amines and chlorophosphines

A catalyst-free synthesis of phosphinic amides from hydroxyl amines and chlorophosphines
N-Benzyl ketimines undergo [3 + 2] cycloaddition with arylacetylenes in KOtBu/DMSO solution to provide 2,3,5-triarylpyrrolines

N-Benzyl ketimines undergo [3 + 2] cycloaddition with arylacetylenes in KOtBu/DMSO solution to provide 2,3,5-triarylpyrrolines
Aldimines undergo nucleophilic addition with diethyl phosphite in the presence of a catalytic amount of zirconium tetrachloride

Aldimines undergo nucleophilic addition with diethyl phosphite in the presence of a catalytic amount of zirconium tetrachloride
Boronic acid catalyzed one-pot reductions of quinolines with Hantzsch ester followed by N-arylations via external base-free Chan-Evans-Lam coupling

Boronic acid catalyzed one-pot reductions of quinolines with Hantzsch ester followed by N-arylations via external base-free Chan-Evans-Lam coupling
Chiral bis(oxazoline)alkynylphosphine ligands can be used in Rh-catalyzed highly regio- and enantioselective allylic amination reactions

Chiral bis(oxazoline)alkynylphosphine ligands can be used in Rh-catalyzed highly regio- and enantioselective allylic amination reactions
Indole and 2-methylindole undergo conjugate addition with electron-deficient olefins in the presence of a catalytic amount of InCl3

Indole and 2-methylindole undergo conjugate addition with electron-deficient olefins in the presence of a catalytic amount of InCl3
Eosin Y catalyzes an efficient photochemical aerobic oxidation of various benzyl alcohols to the corresponding aldehydes or ketones

Eosin Y catalyzes an efficient photochemical aerobic oxidation of various benzyl alcohols to the corresponding aldehydes or ketones
A visible light-promoted divergent cycloaddition of α-diazo esters to hexahydro-1,3,5-triazines provides a series of aziridines and imidazolidines

A visible light-promoted divergent cycloaddition of α-diazo esters to hexahydro-1,3,5-triazines provides a series of aziridines and imidazolidines
A practical method for large-scale acetylation of 1° and 2° alcohols as well as diols has been developed.

A practical method for large-scale acetylation of 1° and 2° alcohols as well as diols has been developed.
A Pd-catalyzed enantioselective aminochlorination of alkenes provides diverse 3-chloropiperidines via a 6-endo cyclization

A Pd-catalyzed enantioselective aminochlorination of alkenes provides diverse 3-chloropiperidines via a 6-endo cyclization
A solvent-free, TfOH-promoted decyanative cyclization enables the synthesis of 2,1-benzisoxazoles in good yields.

A solvent-free, TfOH-promoted decyanative cyclization enables the synthesis of 2,1-benzisoxazoles in good yields.
www.organic-chemistry.org/info/present...
30+ years of synthetic expertise, now delivering building blocks & personalized insights through custom synthesis

www.organic-chemistry.org/info/present...
30+ years of synthetic expertise, now delivering building blocks & personalized insights through custom synthesis
A polystyrene-bound pyridineboronic acid was synthesized and shown to be an efficient, easily recoverable and reusable amidation catalyst.

A polystyrene-bound pyridineboronic acid was synthesized and shown to be an efficient, easily recoverable and reusable amidation catalyst.

