
Interests in data analysis, statistics, open science, clinical trials integrity, corruption, fertility trends and censorship.
Below a bug report which should have been accepted - or the label should at the very least have been fixed.
But hey, if you want, you can buy the data they scrapped on Pubmed 🤔
@brandonstell.bsky.social


Below a bug report which should have been accepted - or the label should at the very least have been fixed.
But hey, if you want, you can buy the data they scrapped on Pubmed 🤔
@brandonstell.bsky.social
openvaet.substack.com/p/pfizerbion...
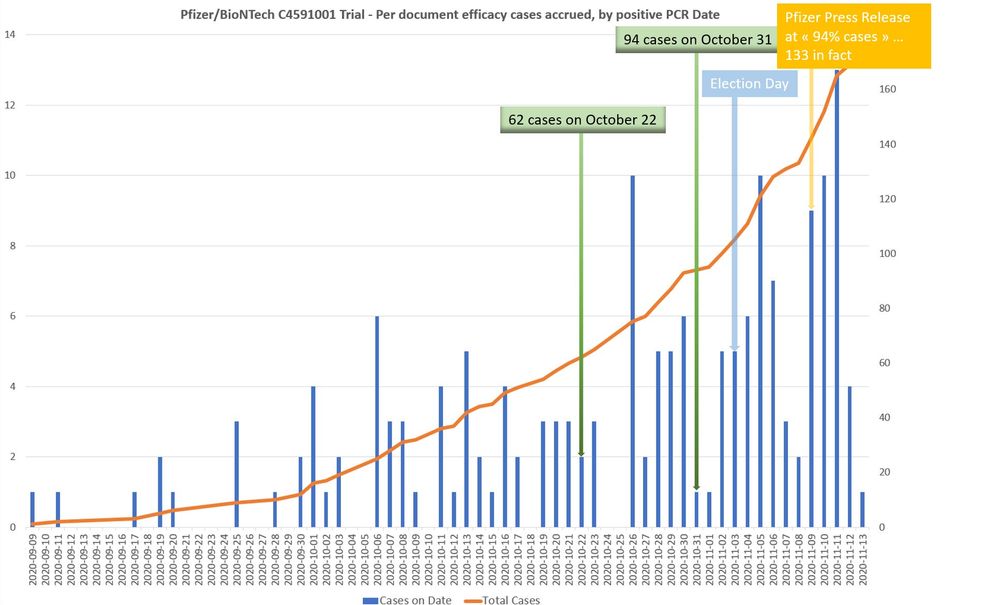
openvaet.substack.com/p/pfizerbion...
Trump did rush the vaccine to score a political win, but we know Pfizer delayed the efficacy announcement to deny him this win 🙄...
openvaet.substack.com/p/pfizerbion...
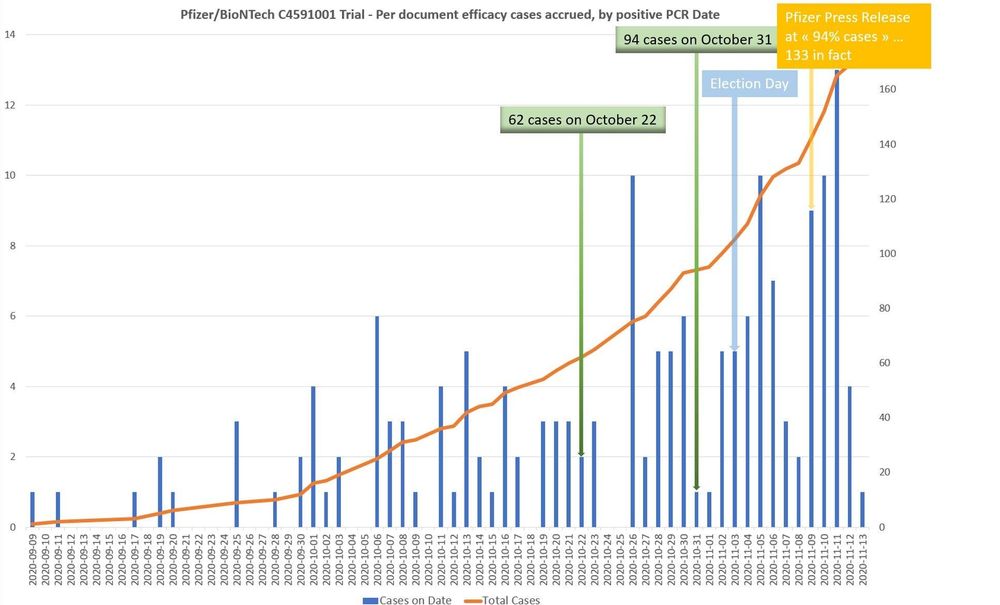
Trump did rush the vaccine to score a political win, but we know Pfizer delayed the efficacy announcement to deny him this win 🙄...
openvaet.substack.com/p/pfizerbion...
File that under "common sense" 👇
open.alberta.ca/publications...
@vikilovesfacs.bsky.social @debunk-the-funk.bsky.social @pauloffit.bsky.social

File that under "common sense" 👇
open.alberta.ca/publications...
@vikilovesfacs.bsky.social @debunk-the-funk.bsky.social @pauloffit.bsky.social
After EUA the placebo recipients were offered the active product.
Obviously you weren't concerned by "having a functioning brain" 🤨
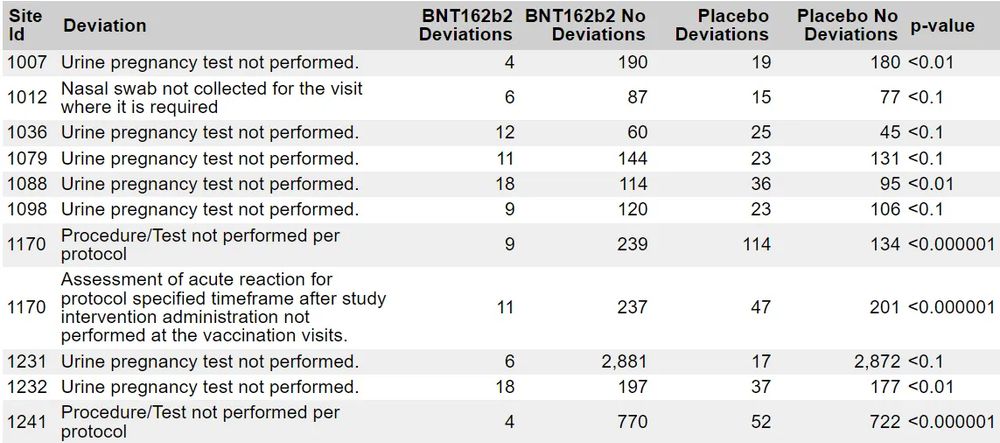
After EUA the placebo recipients were offered the active product.
Obviously you weren't concerned by "having a functioning brain" 🤨
The comparison of process 1 vs process 2 recruitment has been represented on a weekly chart.
19/

The comparison of process 1 vs process 2 recruitment has been represented on a weekly chart.
19/
11/

11/
10/

10/
phmpt.org/wp-content/u...
6/

phmpt.org/wp-content/u...
6/
5/

5/
4/

4/
www.nejm.org/doi/suppl/10...
3/

www.nejm.org/doi/suppl/10...
3/
As a preembule, let's let @pauloffit.bsky.social explain the old rule of the industry : "The process is the product" - with a short extract from "The Curious Case of Science".
1/
As a preembule, let's let @pauloffit.bsky.social explain the old rule of the industry : "The process is the product" - with a short extract from "The Curious Case of Science".
1/

