
Working on combining transition-metal catalysis with data science, machine learning, and high-throughput experimentation to accelerate discovery in synthetic chemistry.
This method utilizes an inexpensive, mild, and soluble base suitable for small & large scales with excellent functional group tolerance across chemical space.
doi.org/10.1021/jacs...

This method utilizes an inexpensive, mild, and soluble base suitable for small & large scales with excellent functional group tolerance across chemical space.
doi.org/10.1021/jacs...


Authors: William Lambert, Stephanie Felten, Nicholas Hadler, N. Ian Rinehart, Rafal Swiatowiec, Gregory Storer, Jeremy Henle, Mark Servos, ...
DOI: 10.26434/chemrxiv-2025-59c10
Authors: William Lambert, Stephanie Felten, Nicholas Hadler, N. Ian Rinehart, Rafal Swiatowiec, Gregory Storer, Jeremy Henle, Mark Servos, ...
DOI: 10.26434/chemrxiv-2025-59c10




Air stable Ir precat for C-H borylation with large scope and 1-pot Borylation-Suzuki, bromination or Chan-Lam
Super paper and will be of high use in med chem #ChemSky
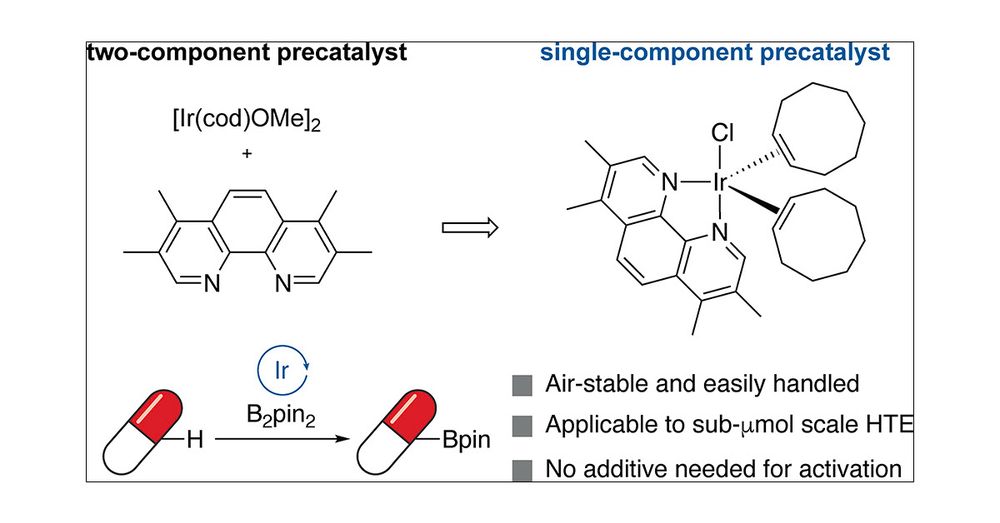
Air stable Ir precat for C-H borylation with large scope and 1-pot Borylation-Suzuki, bromination or Chan-Lam
Super paper and will be of high use in med chem #ChemSky


