doi.org/10.1016/j.dn...
doi.org/10.1016/j.dn...
www.nature.com/articles/s41...
www.science.org/doi/10.1126/...
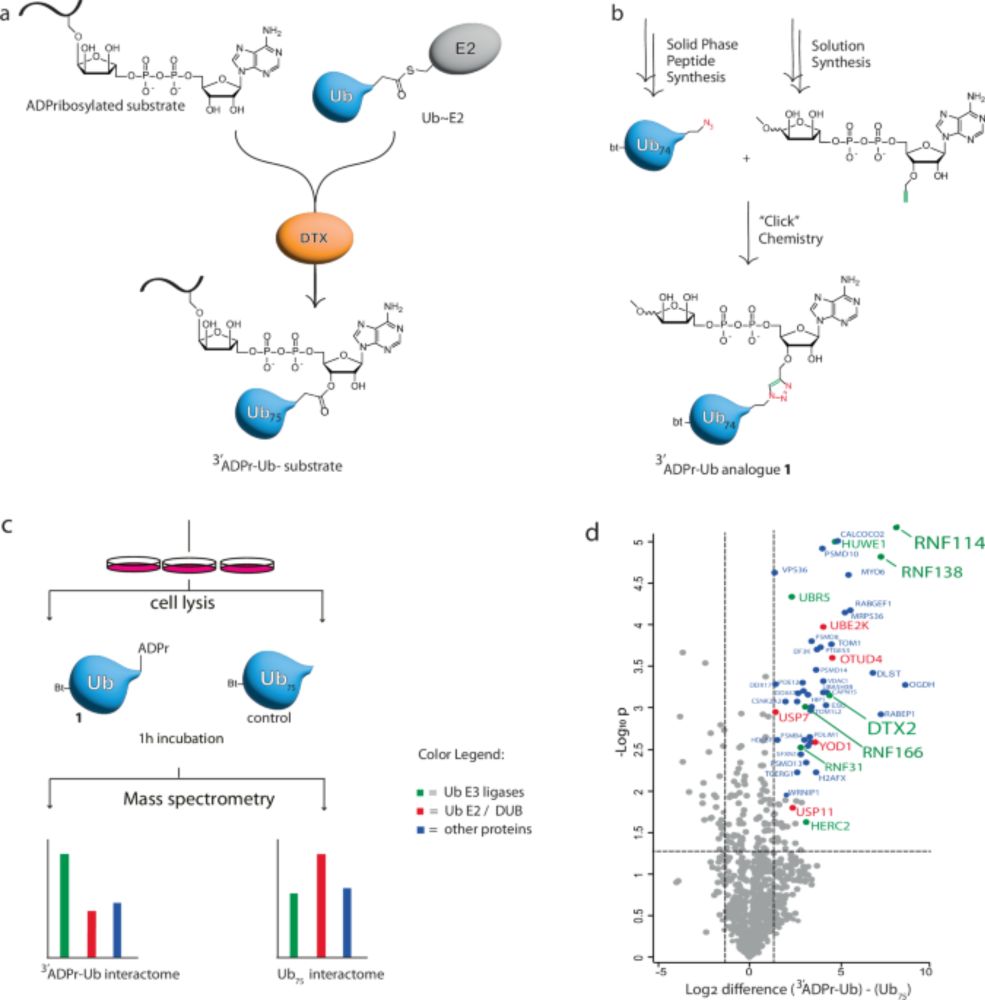
www.nature.com/articles/s41...
www.science.org/doi/10.1126/...
www.nature.com/articles/s41...

www.nature.com/articles/s41...

