
https://www.jose-silva-lab.com/ 🇵🇹🇨🇳🇬🇧
Guangzhou/Cantāo 🌴☀️, China中国
Visit the lab website for free access to our study (under publications section)
Visit the lab website for free access to our study (under publications section)









www.cell.com/cell-stem-ce...
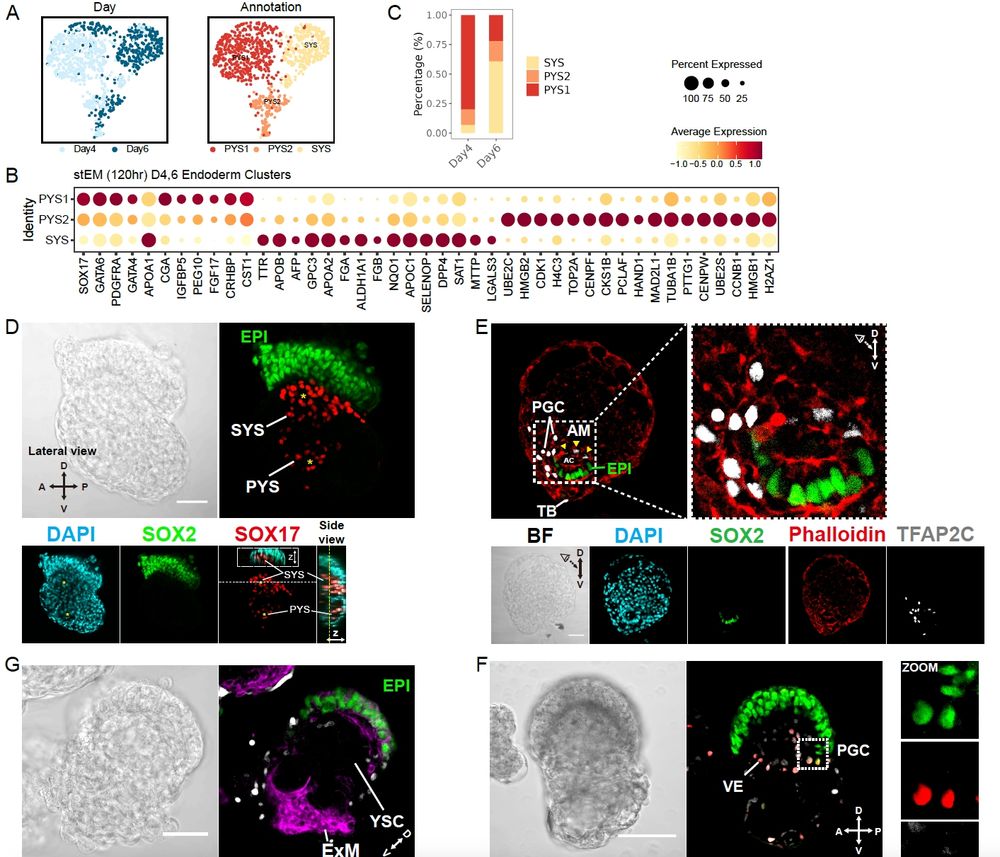
However, we thought this could be paralleling early embryo development and have since been exploring it to generate embryo models.

www.cell.com/cell-stem-ce...
However, we thought this could be paralleling early embryo development and have since been exploring it to generate embryo models.

authors.elsevier.com/sd/article/S...
authors.elsevier.com/sd/article/S...

