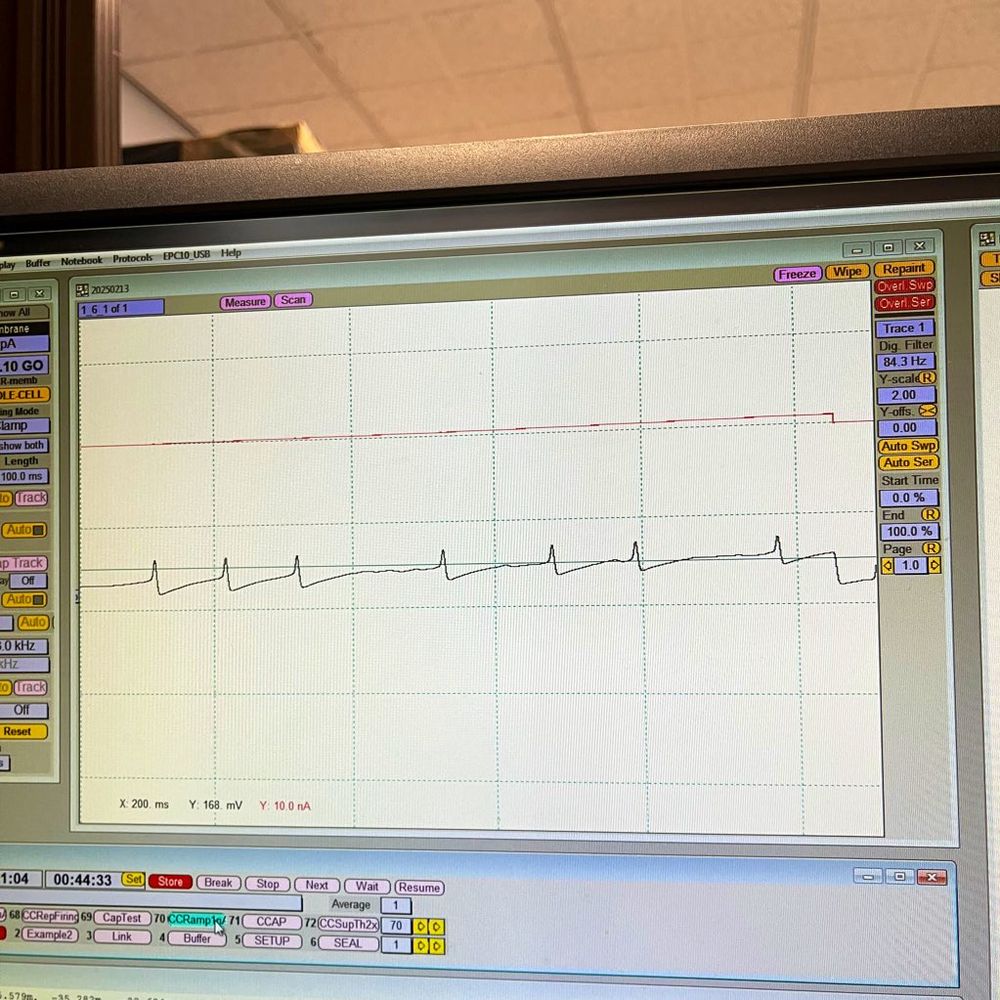
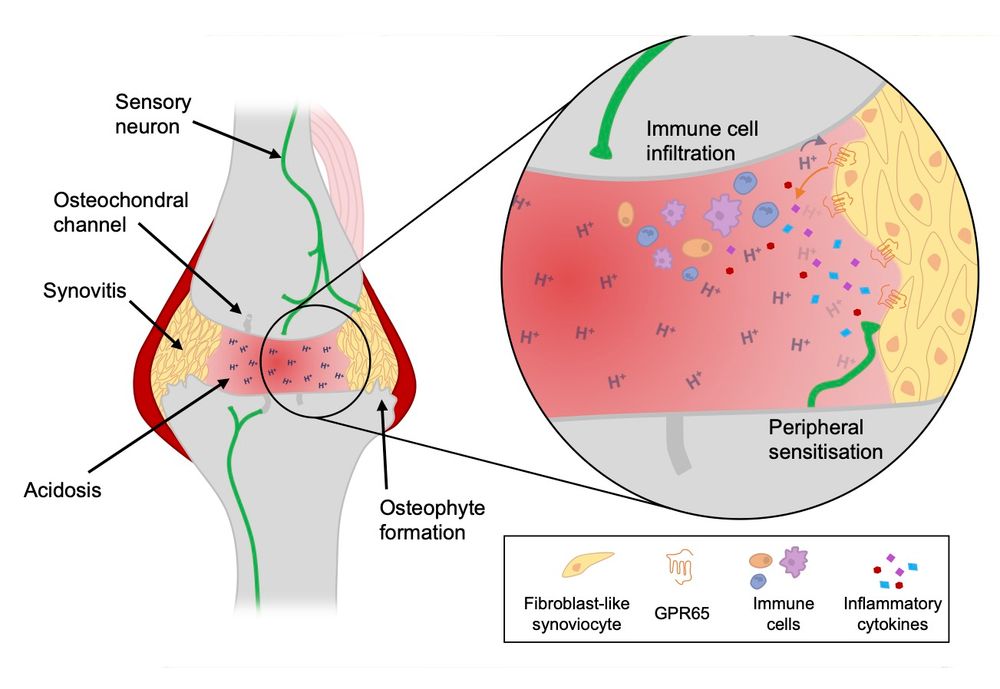
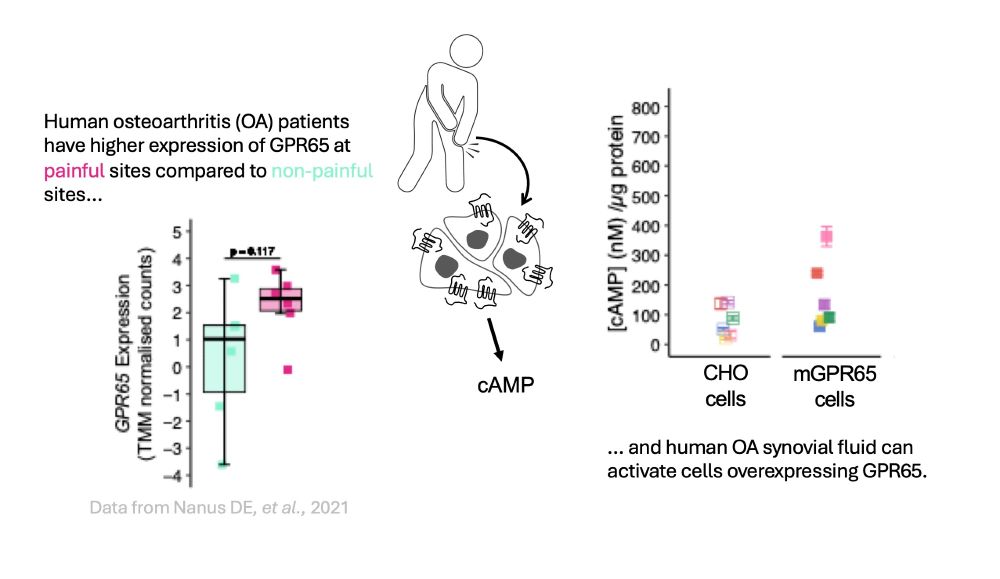
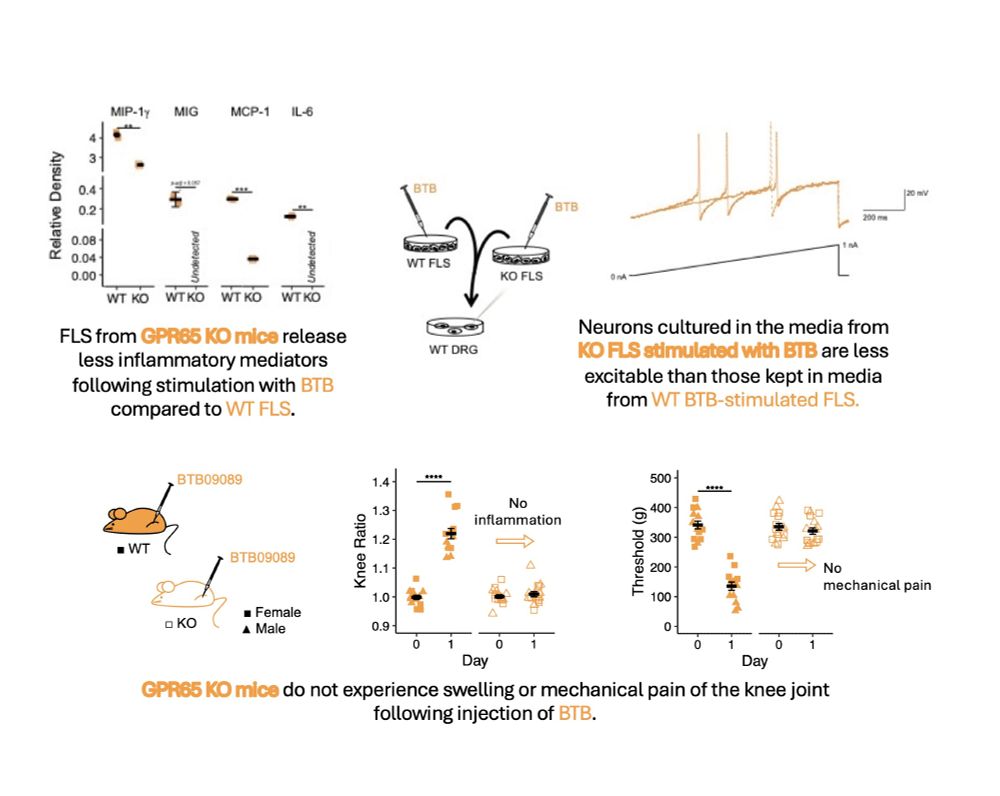
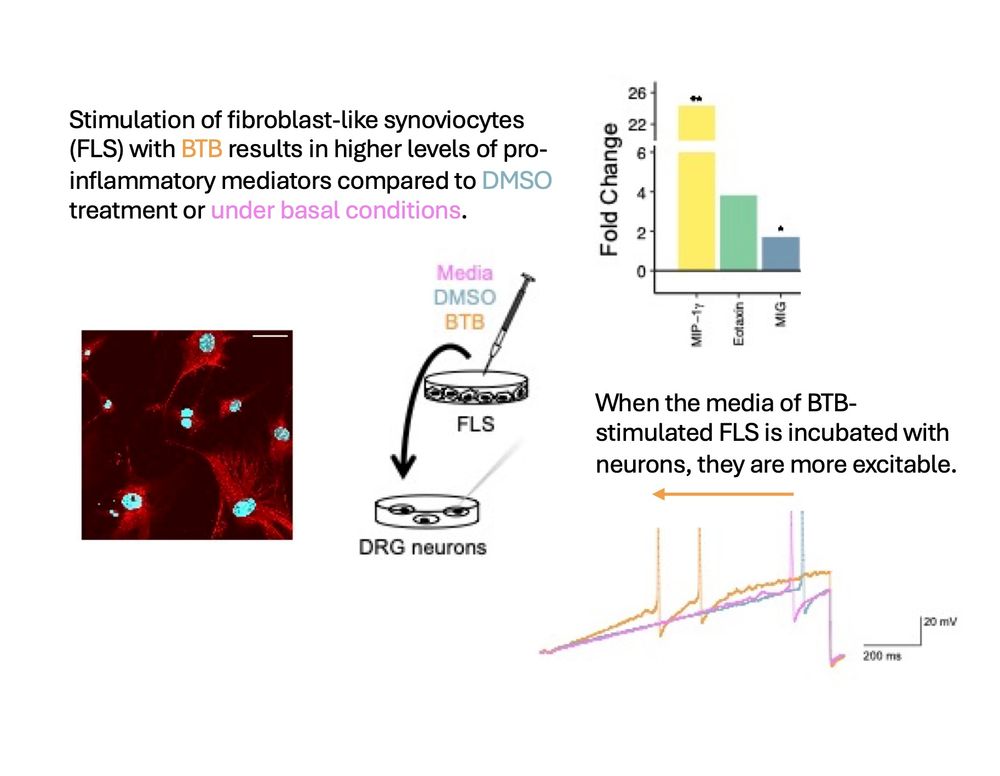
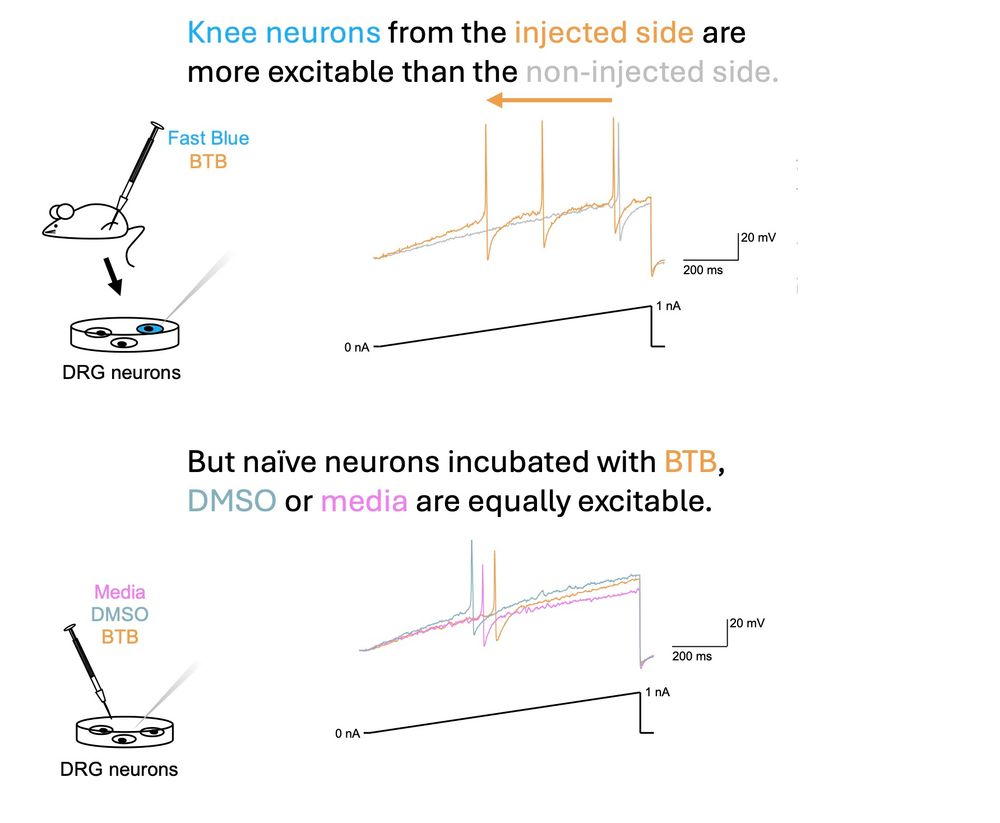
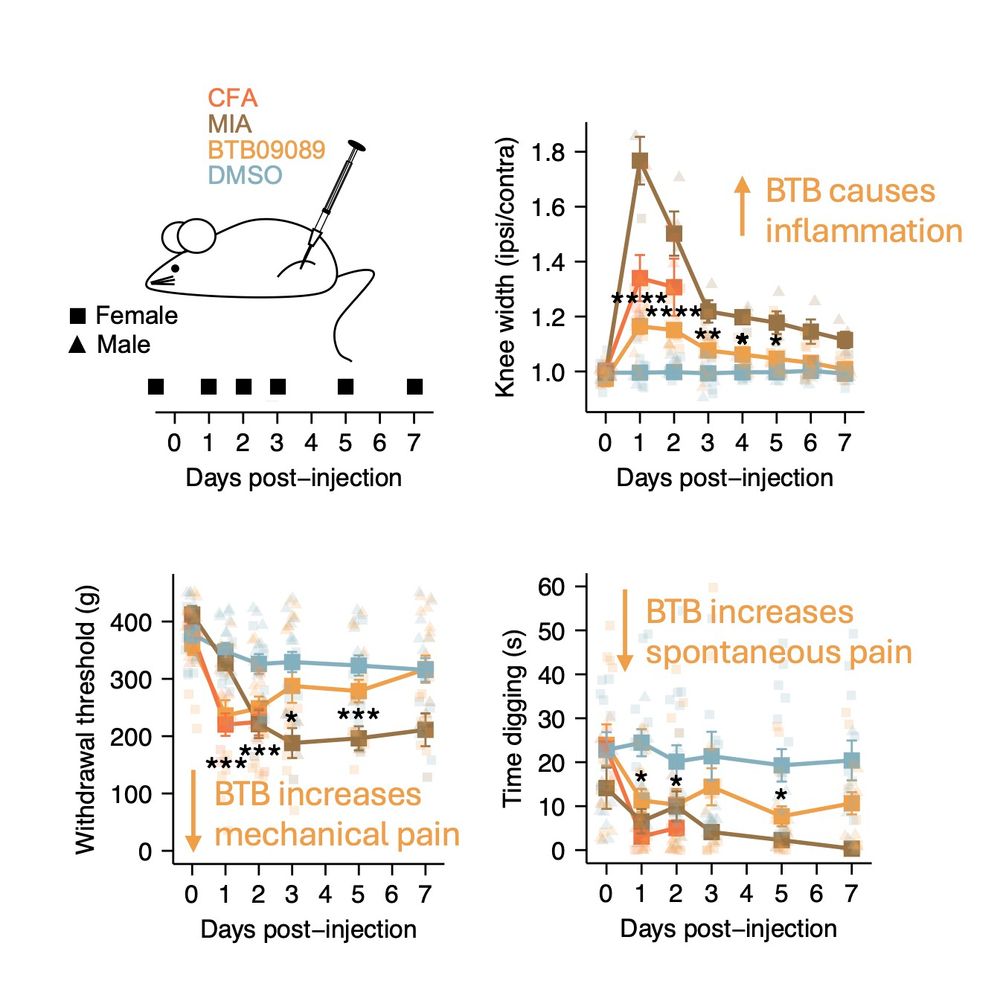
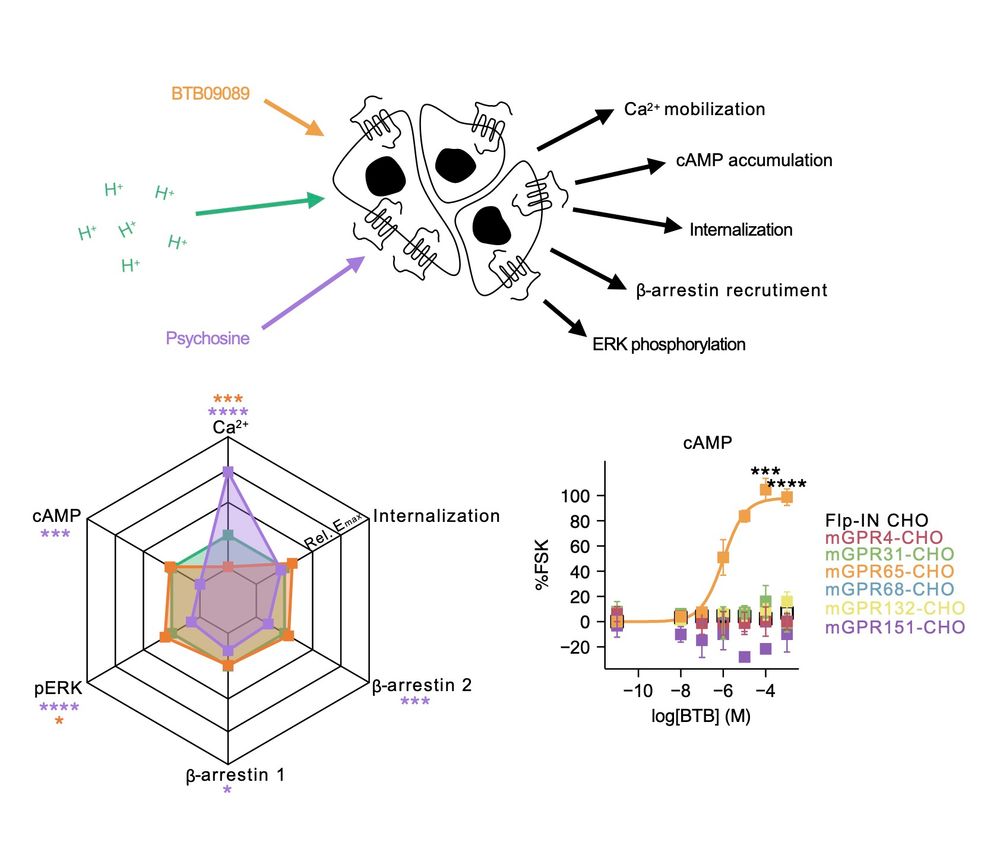
on “Molecular Mechanisms Underlying Inflammatory Pain.”
If you are working on, or planning a suitable manuscript, I would be delighted if you would consider submitting it to our issue.
mdpi.com/journal/cell...

on “Molecular Mechanisms Underlying Inflammatory Pain.”
If you are working on, or planning a suitable manuscript, I would be delighted if you would consider submitting it to our issue.
mdpi.com/journal/cell...
Proud to watch my Pegasus Scholars students presenting on targeted cancer therapies today, after a week exploring the formation, functions, faults and fixing of proteins! 🔬
Glad to support Robinson College’s excellent widening participation initiation for another year! 👨🏫

Proud to watch my Pegasus Scholars students presenting on targeted cancer therapies today, after a week exploring the formation, functions, faults and fixing of proteins! 🔬
Glad to support Robinson College’s excellent widening participation initiation for another year! 👨🏫